IGCSE Physics Past Years Exam Questions: Radioactivity and particles 2023-24
We analysed the Pearson Edexcel International GCSE past papers and grouped the questions by topic. Here, you will find questions relating to the topic – Radioactivity and particles. Use these to familiarise, practice and prepare for your IGCSE Physics examination.
You can find past years exam questions in the same topic for earlier papers below:
What you need to know
Use the list below as a quick recap for what you need to know before attempting the past year exam questions under this topic. This is based on Edexcel International GCSE in Physics (4PH1) specification with first teaching Sept 2017 and first examination June 2019.
Paper 1 and 2: (7) Radioactivity and particles
Paper 1 covers all the topics except where it is marked “Paper 2 only” while Paper 2 covers all topics.
A. Units
- becquerel (Bq), centimetre (cm), hour (h), minute (min) and second (s)
B. Radioactivity
- describe the structure of the atom in terms of protons, neutrons and electrons
- know the terms atomic(proton) number, mass (nucleon) number and isotope
- know that alpha (α) particles, beta (β–) particles and gamma (γ) rays are ionising radiations emitted from unstable nuclei in a random process
- describe the properties of α and ß particles and γ rays in terms of penetrating power and ability to ionise
- describe the effect on the atomic and mass number of nuclei caused by the emission of the four main types of radiation (alpha, beta, gamma and neutron radiation)
- understand how to balance a nuclear equation in terms of mass and charge
- know that ionising radiations may be detected by a Geiger-Müller tube or photographic film
- explain the sources of background (ionising) radiation from Earth and space
- know that the activity of a radioactive source decreases over time and is measured in becquerels (Bq)
- definition of the term half-life and use the concept to carry out calculations on activity, including graphical method
- uses of radioactivity in industry and medicine
- the difference between contamination and irradiation
- the dangers of ionising radiation: mutation in living organisms, damage cells and tissue, problems from the disposal of radioactive waste and how to reduce the risks.
C. Fission and fusion
- know that nuclear reactions, including fission, fusion and radioactive decay can be sources of energy
- that a nucleus of U-235 can be split by collision with a neutron (fission) and that the process releases kinetic energy
- that the fission of U-235 produces two radioactive daughter nuclei and a small number of neutrons
- describe how a chain reaction of fissions can be set up if the neutrons produced by one fission strike other U-235 nuclei
- describe the role played by control rods, moderator, and shielding in a nuclear reactor
- explain the difference between nuclear fission and nuclear fusion
- describe nuclear fusion as the creation of larger nuclei resulting in a loss of mass from smaller nuclei accompanied by a release of energy
- know that fusion is the energy source for stars
- explain why fusion does not happen at low temperatures and pressures, due to electrostatic repulsion of protons.
January 2023 Paper 1P Q4
4 A teacher investigates the count rate detected from a radioactive source. (a) (i) State one source of background radiation. (1)
(ii) Describe how the teacher could measure the count rate from a radioactive source and correct the count rate for background radiation. (4)
(b) The teacher places a piece of lead sheet between the radioactive source and a radiation detector.
The teacher determines the corrected count rate from the radioactive source three times and calculates the mean.
They repeat this process using different thicknesses of lead sheet. The table shows their results.
| Thickness of lead in mm | Count rate in Bq | |||
| trial 1 | trial 2 | trial 3 | mean | |
| 0.0 | 480 | 504 | 469 | 484 |
| 2.0 | 374 | 337 | 357 | 356 |
| 4.0 | 247 | 239 | 229 | 238 |
| 6.0 | 141 | 154 | 148 | |
| 8.0 | 110 | 104 | 131 | 115 |
| 10.0 | 88 | 91 | 85 | 88 |
(i) Calculate the mean count rate when the thickness of lead is 6.0 mm. (2)
mean count rate = …………………………………………………….. Bq
(ii) Plot a graph of mean count rate against thickness of lead. (3)
(iii) Draw the curve of best fit. (1)
(iv) When there is not a sheet of lead between the radioactive source and the radiation detector, the mean count rate is 484 Bq.
Use the graph to determine the thickness of lead needed to reduce the mean count rate by 25%. (2)
thickness = …………………………………………………….. mm
(c) The radioactive source emits only one type of radiation.
Explain which type of radiation this radioactive source emits. (2)
(Total for Question 4 = 15 marks)
January 2023 Paper 1PR Q5
5 The photograph shows the nuclear fusion reactor called ITER.

(a) Describe the difference between nuclear fusion and nuclear fission. (2)
(b) At ITER, hydrogen-3 and hydrogen-2 will be fused into helium and another particle labelled X.
(i) Complete the nuclear equation for the fusion of hydrogen-3 and hydrogen-2. (2)
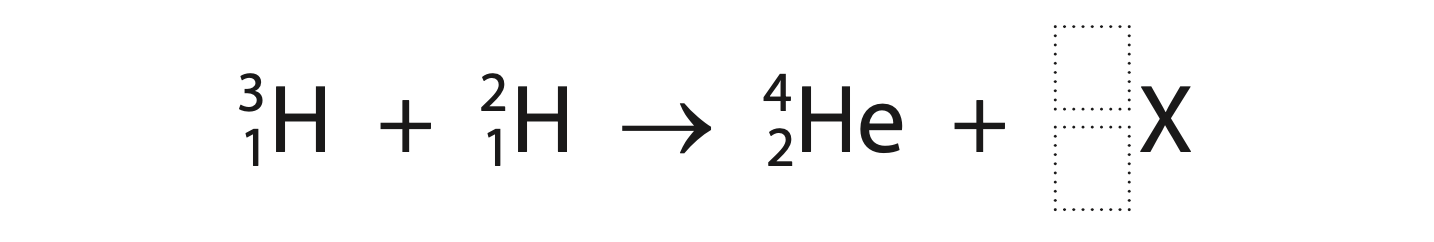
(ii) Discuss potential safety advantages of a fusion reactor compared with a fission reactor.
You should refer to the products of this fusion reaction and to the fission reaction of uranium. (3)
(c) The half-life of a radioactive isotope is 12 years.
The activity of a sample of this isotope is 120 kBq.
Calculate the activity of this sample after 48 years. (3)
activity = …………………………………………………….. kBq
(Total for Question 5 = 10 marks)
January 2023 Paper 1PR Q9
9 A teacher demonstrates the penetrating ability of alpha, beta and gamma radiation from some radioactive sources.
(a) (i) State a precaution the teacher should take to make sure they are working safely with the radioactive sources. (1)
(ii) State the name of a detector the teacher could use to detect the radiation from each source. (1)
(b) Draw crosses (×) in the table to show which type of radiation cannot penetrate each material in the table. (3)
| Material | |||
| Type of radiation | 10 cm of air | 2 cm of aluminium | 10 cm of lead |
| alpha | |||
| beta | |||
| gamma | |||
(c) An alpha particle of mass 6.6 × 10–27 kg travelling at a speed of 2.1 × 107 m/s hits a sheet of paper.
(i) Calculate the kinetic energy (KE) of the alpha particle. (3)
KE = …………………………………………………….. J
(ii) State the work done on the alpha particle when its speed is reduced to 0 m/s by the sheet of paper. (1)
work done = …………………………………………………….. J
(iii) State which energy store of the paper increases when the alpha particle is stopped.
(Total for Question 9 = 10 marks)
January 2023 Paper 2P Q3
3 Kori Nuclear Power Plant in South Korea is one of the world’s largest nuclear fission power stations.

(a) The reactors at Kori use nuclear fission to generate electricity.
The products released during nuclear fission have high energy in their kinetic store.
Give a product of nuclear fission. (1)
(b) Give two disadvantages of using nuclear fission to generate electricity. (2)
(c) Kori has a maximum power output of 7.49 × 109 W.
(i) State what is meant by the term power. (1)
(ii) Calculate the minimum time taken for Kori to transfer 6.47 × 1014 J of energy.(3)
minimum time = …………………………………………………….. s
(Total for Question 3 = 7 marks)
January 2023 Paper 2P Q1
1. The photograph shows a person using a roll of plastic wrapping to cover a plate of food.
The plastic wrapping sticks to the plate due to electrostatic charges.

The passage explains why the plastic wrapping sticks to the plate. Use words from the box to complete the passage.
Each word may be used once, more than once, or not at all.
| attract electrons negative neutral neutrons positive protons repel |
attract electrons negative neutral neutrons positive protons repel
The person pulls a layer of plastic wrapping from the roll.
Forces between the layers of wrapping transfer particles called …………………………………………………….. from one layer to another layer.
The layer gaining these particles acquires a …………………………………………………….. charge. The layer losing these particles acquires a …………………………………………………….. charge.
The negatively charged layer of wrapping repels …………………………………………………….. in the plate, leaving a positive charge in the plate where it touches the plastic wrapping.
The wrapping and plate …………………………………………………….. due to them having opposite charges.
(Total for Question 1 = 5 marks)
January 2023 Paper 2PR Q6
6. This question is about the use of water in central heating systems.
(a) A student does an investigation to find the specific heat capacity of water. This is the list of equipment they use.
- heater with a power output of 50 W
- power supply
- beaker
- water
- thermometer
- stopwatch
- connecting leads
- balance
Describe an investigation the student could use to find the specific heat capacity of water.
You may draw a diagram to help your answer. (5)
(b) The diagram shows a simplified central heating system viewed from above.
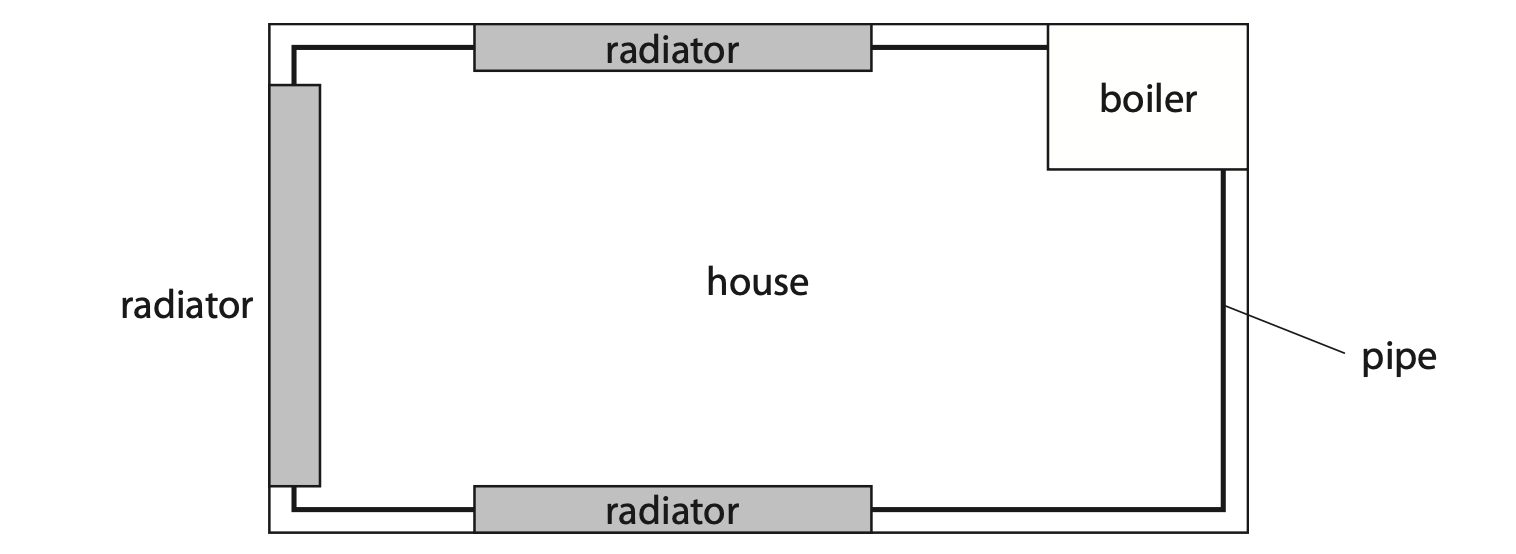
Pipes transport hot water around a house to radiators and back to the boiler.
The boiler heats water from 16 °C to 65 °C.
(i) Calculate the energy transferred from the boiler to 75 kg of water to raise the temperature of the water from 16 °C to 65 °C.
[for water, specific heat capacity = 4200 J/kg °C] (3)
energy = …………………………………………………….. J
(ii) The radiators transfer energy from the water to the air in the house.
The temperature of the water in the heating system decreases by 4 °C due to heat transferred to the air.
This causes the air in the house to increase in temperature by 15 °C.
The mass of air in the house and the mass of water in the heating system are approximately the same.
Explain why there is a larger temperature change in the air. (3)
(Total for Question 6 = 11 marks)
June 2023 Paper 1P Q1
1. This is a question about radioactivity.
(a) Which of these is the unit of activity? (1)
A becquerel
B kilogram
C newton
D pascal
(b) Which of these is the correct description of the term half-life? (1)
A time taken for the activity of a substance to halve
B half of the time taken for the mass of a substance to decay
C time taken for the activity to decay completely
D time taken for the mass of a substance to decay twice
(c) A teacher demonstrates how the activity of a radioactive sample changes with time.
(i) The box gives the names of different pieces of equipment.
| Ruler, stopwatch, balance, newton meter, protractor, GM tube, voltmeter, ammeter |
Complete the sentences using words from the box. (2)
The teacher measures time with a …………………………………………………….. .
The teacher measures the count rate with a …………………………………………………….. and a counter.
(ii) The graph shows the teacher’s results.
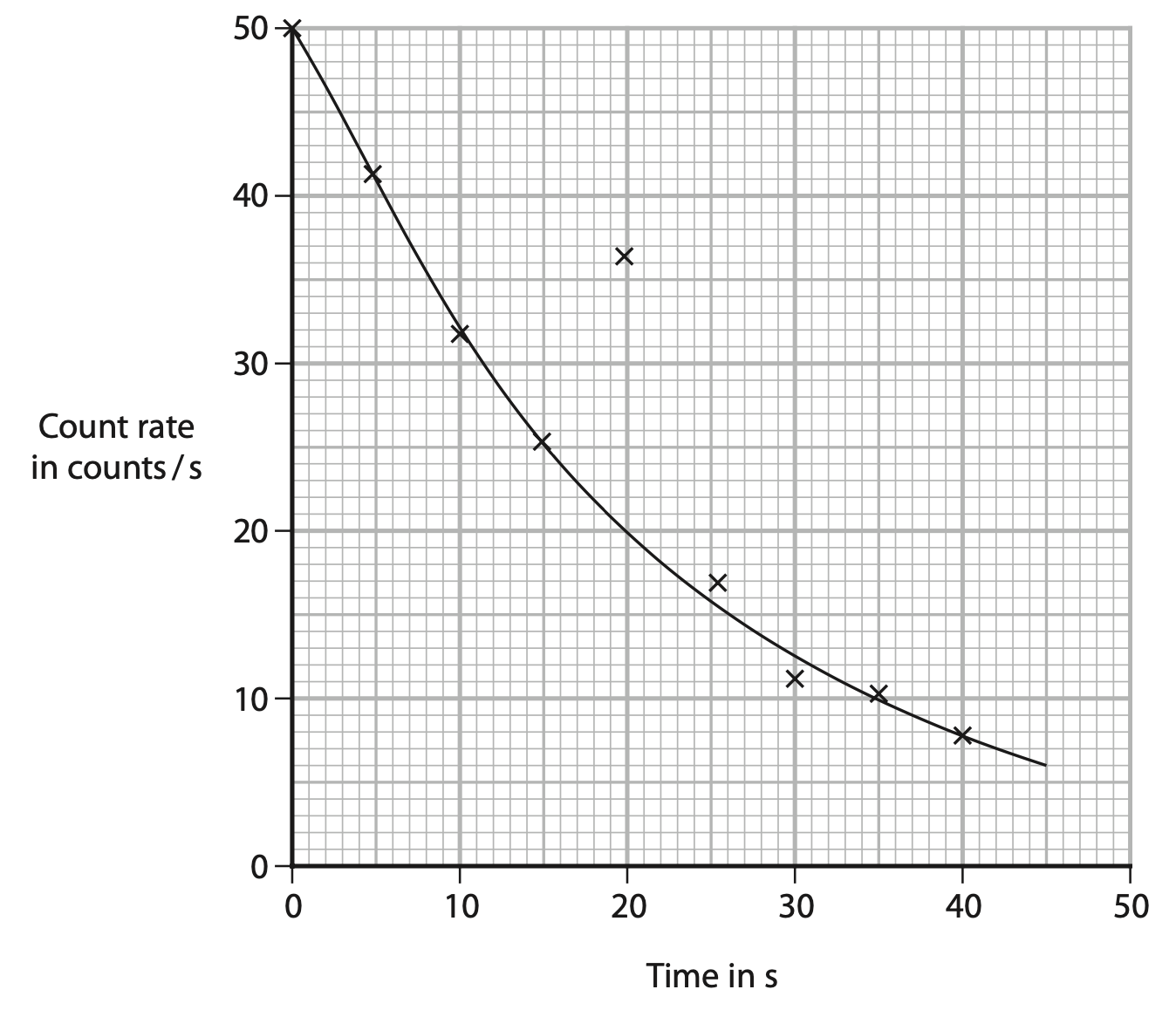
Draw a circle around the anomalous result. (1)
(iii) Use the graph to determine the half-life of the radioactive sample (2)
half-life = …………………………………………………….. s
(iv) Give a reason why the teacher should not expect the data points to lie exactly on the curve of best fit (1)
(Total for Question 1 = 8 marks)
June 2023 Paper 1PR Q7
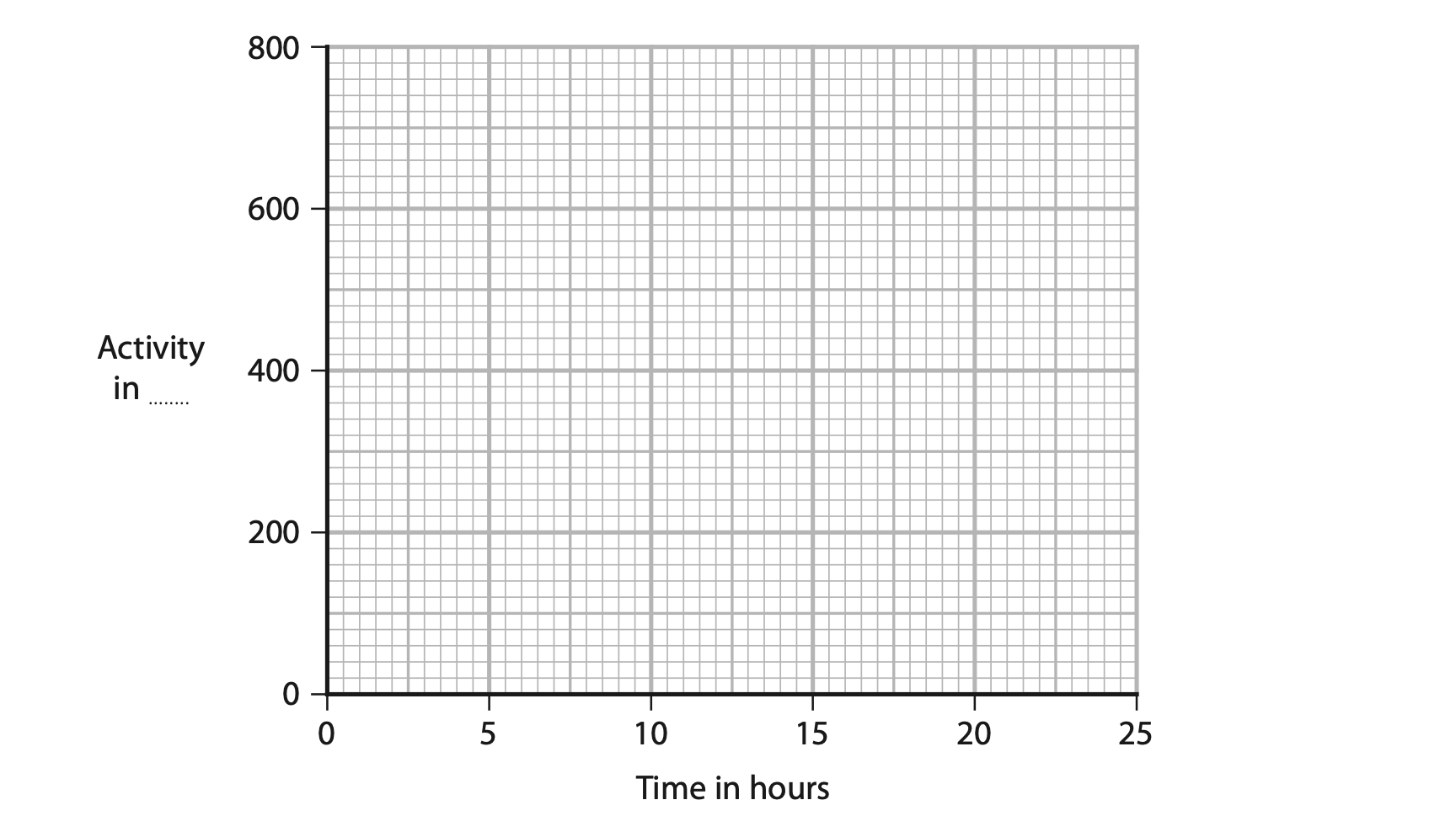
7. Protactinium is an element with several different radioactive isotopes.
(a) Protactinium-234 has a half-life of 6.7 hours.
A sample of protactinium-234 has an initial activity of 800 units.
(i) Give a suitable unit for activity. (1)
(ii) On the axes below, sketch a graph for the decay of the sample of
protactinium-234 during its first three half-lives. (3)
(iii) When protactinium-234 undergoes beta (β–) decay it becomes uranium-234.
The incomplete nuclear equation shows this process.
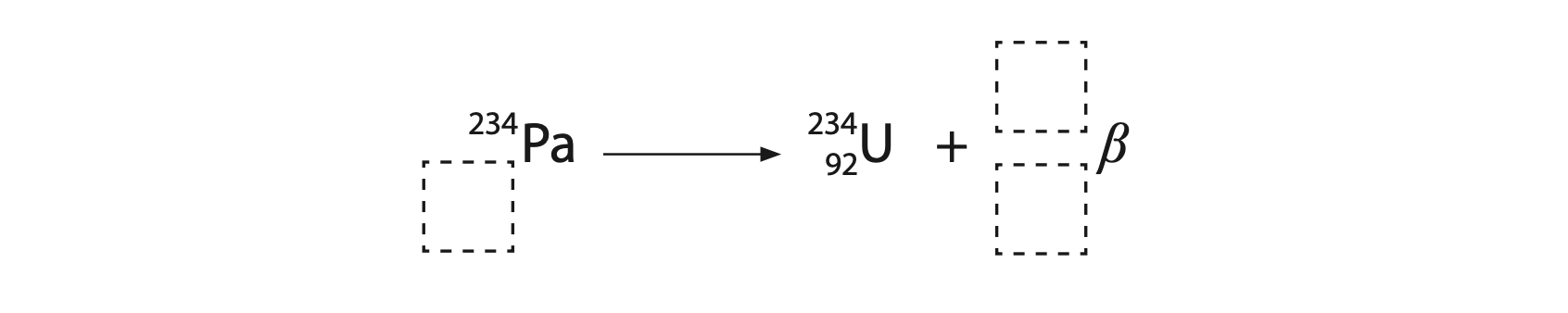
Complete the nuclear equation to show the beta decay of protactinium-234.
Write your answers in the dashed boxes. (2)
(b) A student suggests an experiment to determine the type of radiation emitted by a different isotope of protactinium, protactinium-231.
This is the suggested method.
| Step 1 | connect a suitable radiation detector to a radiation counter |
| Step 2 | place a source of protactinium-231 at a fixed distance of 3 cm from the radiation detector |
| Step 3 | record the count of detected radiation for a time of one minute |
| Step 4 | place a sheet of paper between the source and detector |
| Step 5 | record the count of detected radiation for a time of one minute |
| Step 6 | repeat Steps 4 and 5 using a sheet of aluminium and then a sheet of lead instead of the sheet of paper |
The table shows the results of the investigation when it is done by a teacher.
| Material between source and detector | Count |
| no material | 261 |
| paper | 14 |
| aluminium | 11 |
| lead | 13 |
(i) Which of these is the dependent variable in the investigation? (1)
A count measured by the detector
B distance between source and detector
C material between source and detector
D time the count is measured
(ii) The student’s method does not allow for background radiation.
Describe how the student’s method should be modified to allow for background radiation. (3)
(iii) Describe how the student’s method could be modified to improve the reliability of the results. (2)
(iv) Evaluate the data from the experiment to conclude the type of radiation emitted by protactinium-231. (3)
(Total for Question 7 = 15 marks)
June 2023 Paper 2P Q3
3. The diagram shows the inside of an oscilloscope.
Electrons leave the electron supply and accelerate towards the screen.
The stream of electrons hits the screen.
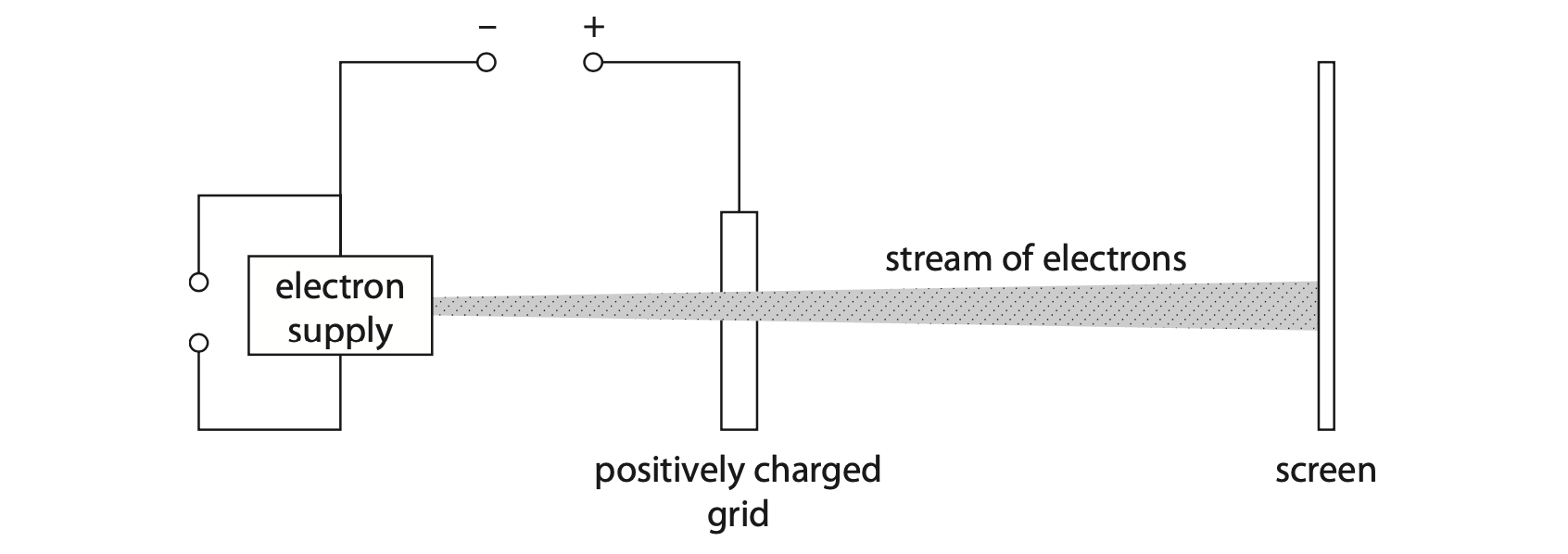
(a) The grid is connected to the positive terminal of a high voltage power supply.
(i) Explain, in terms of the movement of particles, how the grid has become positively charged. (2)
(ii) State the formula linking kinetic energy, mass and speed. (1)
(iii) Calculate the speed of an electron when it has 1.3 × 10 –15 J of kinetic energy. The mass of an electron is 9.1 × 10 –31 kg. (3)
speed = …………………………………………………….. m/s
(b) The stream of electrons spreads out as it travels towards the screen.
Explain why the electrons in the stream move apart from each other. (2)
(c) The oscilloscope can cause the electrons to move in a circle.
This produces a circular pattern on the screen.

(i) Use the scale to determine the radius of the circle. (1)
radius = …………………………………………………….. cm
(ii) The time taken for the electrons to complete one orbit of the circle is 24 ms.
Calculate the orbital speed of the electrons. (3)
Use the formula
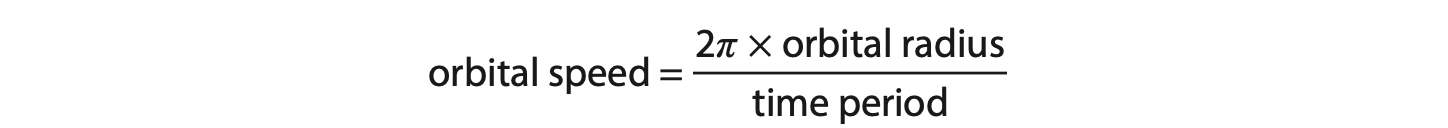
orbital speed = …………………………………………………….. m/s
(Total for Question 3 = 12 marks)
June 2023 Paper 2P Q5
5. The diagram shows some of the components of a nuclear fission reactor.
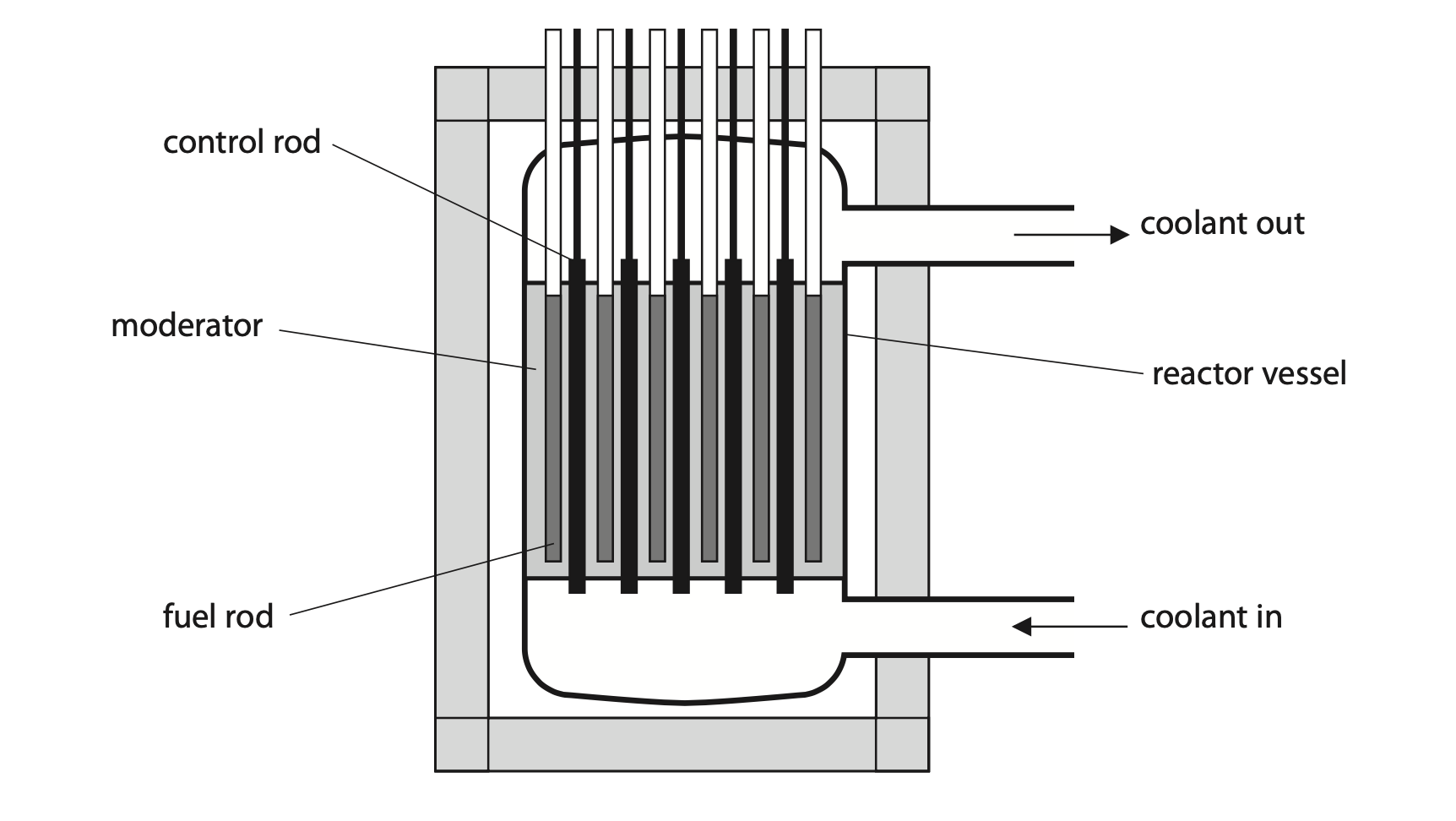
(a) Describe how a chain reaction occurs in a nuclear fission reactor. (3)
(b) Describe the role of the control rods in a nuclear fission reactor. (2)
(c) Describe how the process of nuclear fission is different from the process of nuclear fusion. (2)
(d) (i) Which of these is the name of the nuclear process that is the main energy source for stars? (1)
A alpha decay
B fission
C fusion
D gamma decay
(ii) Give a reason why the nuclear process in stars does not happen at low temperatures and pressures. (1)
(Total for Question 5 = 9 marks)
November 2023 Paper 1P Q7
7. This question is about using carbon dating to find the age of pieces of wood. (a) The equation shows how carbon-14 forms in the atmosphere.
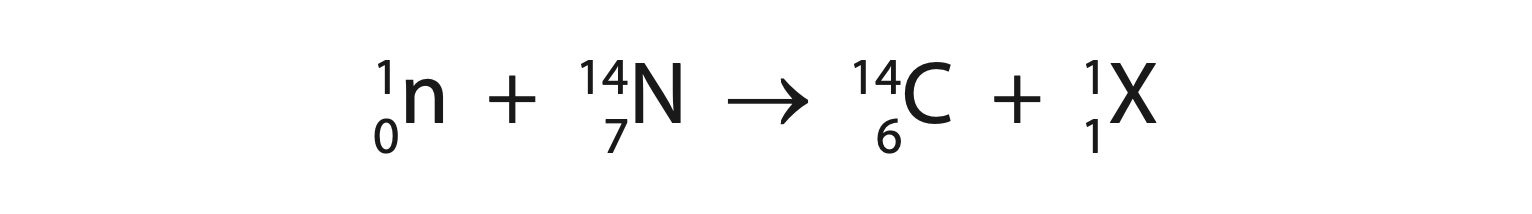
(i) State the name of particle X. (1)
(ii) Carbon-14 decays by beta decay.
State what happens to the number of protons and the number of neutrons in a carbon-14 nucleus when it decays. (2)
(b) A scientist determines how the percentage of carbon-14 remaining in a sample of wood changes with time.
The table shows the scientist’s data.
| Percentage (%) of carbon-14 remaining | Age of sample in years |
| 30 | 9900 |
| 40 | 7500 |
| 50 | 5700 |
| 60 | 4200 |
| 70 | 2900 |
(i) Plot the scientist’s data on the grid. (3)
(ii) Draw the curve of best fit. (1)
(iii) Use the graph to determine the age of a sample of wood that has 36% of its carbon-14 remaining. (2)
age = …………………………………………………….. years
(c) Carbon-14 dating is inaccurate for samples of wood produced after 1950.
This is because the concentration of carbon-14 in the atmosphere greatly increased due to nuclear weapons testing.
(i) A tree absorbs carbon-14 during its lifetime.
A student suggests that trees grown after 1950 are contaminated with carbon-14.
Give a reason why the student’s suggestion is correct. (1)
(ii) Explain how nuclear weapons testing affects the determination of the age of a
sample of wood produced after 1950. (2)
(Total for Question 7 = 12 marks)










