IGCSE Physics Past Years Exam Questions: Solids, Liquids and Gases 2023-24
We analysed the International GCSE past papers and grouped the questions by topic. Here, you will find questions relating to the topic – Solids, liquids and gases. Use these to familiarise, practice and prepare for your IGCSE Physics examination.
You can find earlier questions in the same topic below:
What you need to know
Use the list below as a quick recap for what you need to know before attempting the past year exam questions under this topic. This is based on Edexcel International GCSE in Physics (4PH1) specification with first teaching Sept 2017 and first examination June 2019.
Paper 1 and 2: (5) Solids, liquids and gases
Paper 1 covers all the topics except where it is marked “Paper 2 only” while Paper 2 covers all topics.
A. Units
- degree Celsius (°C), Kelvin (K), joule (J), kilogram (kg), kilogram/metre3 (kg/m3), metre (m), metre2 (m2), metre3 (m3), metre/second (m/s), metre/second2 (m/s2), newton (N) and pascal (Pa)
- use the following unit: joules/kilogram degree Celsius (J/kg °C)
B. Density and pressure
- relationship between density, mass and volume. ρ = m/V
- investigate density using direct measurements of mass and volume.
- relationship between pressure, force and area. p = F/A
- pressure at a point in gas or liquid acts equally in all directions.
- relationship between pressure difference, height, density and gravitational field strength. p=h×ρ×g
C. Change of state (Paper 2 only)
- that heating a system will change the energy stored in that system. (raise its temperature or product changes of state)
- changes that occur when a solid melts to liquid and when a liquid boils or evaporates to gas.
- describe the arrangements of atoms in solids, liquids and gases.
- temperature-time graph to show a constant temperature during a change of state.
- specific heat capacity of a substance is the energy required to change the temperature of 1kg of a substance by 1°C. (J/kg °C)
- equation relating to the change in thermal energy to mass, specific heat capacity and temperature change. ΔQ = m × c × ΔT
- investigate the specific heat capacity of materials including water.
D. Ideal gas molecules
- how molecules in a gas have random motion and exert a pressure on the sides of their container
- understand why there is absolute zero at -273°C.
- describe Kelvin scale of temperature and convert between Kelvin and Celsius scales.
- increase in temperature results in increase in average speed of molecules.
- Kelvin temperatures of a gas are directly proportional to the average kinetic energies of its molecules.
- explain for a fixed mass of mass, the qualitative relationship between:
- pressure and volume at constant temperature;
- pressure and kelvin temperature at constant volume.
- the relationship between the pressure and kelvin temperature of a fixed mass of gas at constant volume. P1/T1 = P2/T2
- relationship between pressure and volume of a fixed mass of mass at constant temperature. P1V1=P2V2
January 2023 Paper 1P Q11
11 (a) Diagram 1 represents the atoms of a gas inside a container.
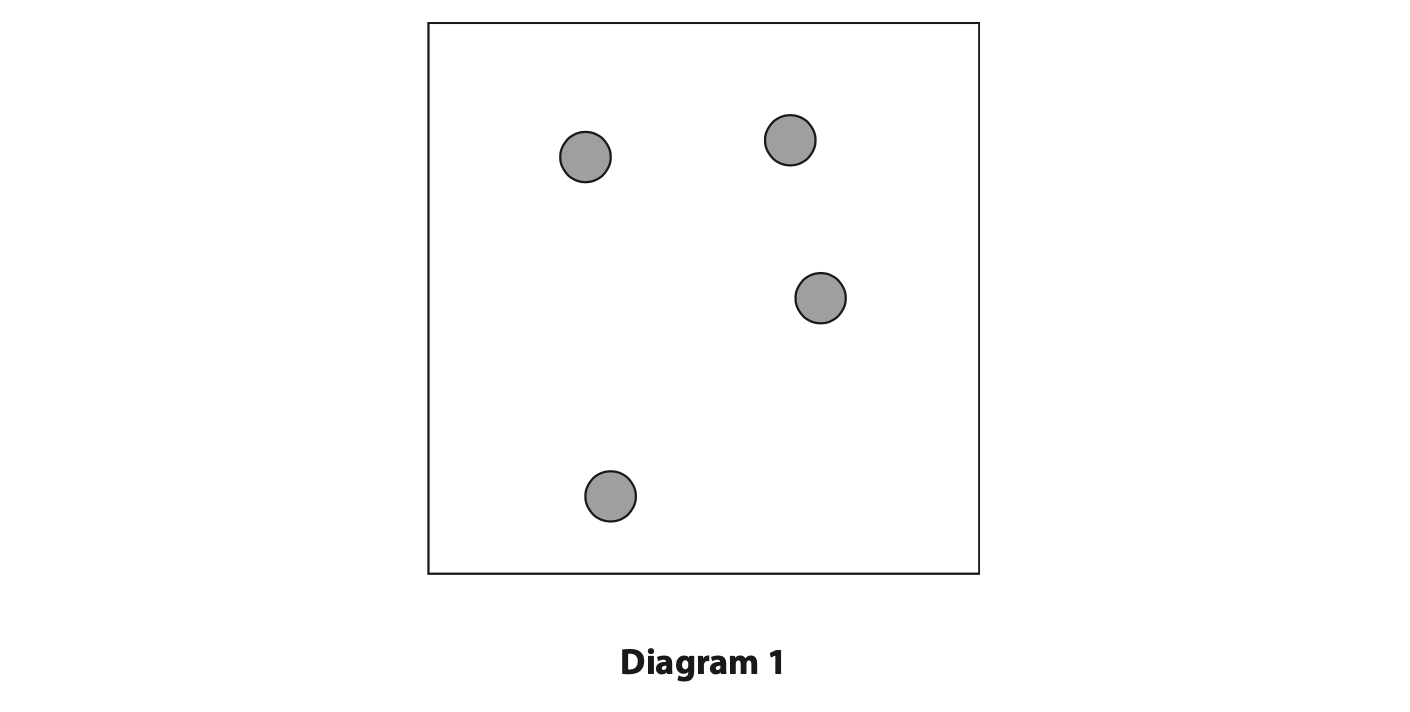
(i) Explain how the atoms exert a pressure on the walls of the container. (3)
(ii) Explain why the pressure of the gas in the container decreases as its temperature decreases.
The volume of the container does not change. (2)
(b) Diagram 2 shows a device called a magneto-optical trap (MOT).
Physicists use the device to cool gases to extremely low temperatures.
The MOT uses laser beams and magnetic fields to trap a small collection of atoms with extremely small kinetic energies.
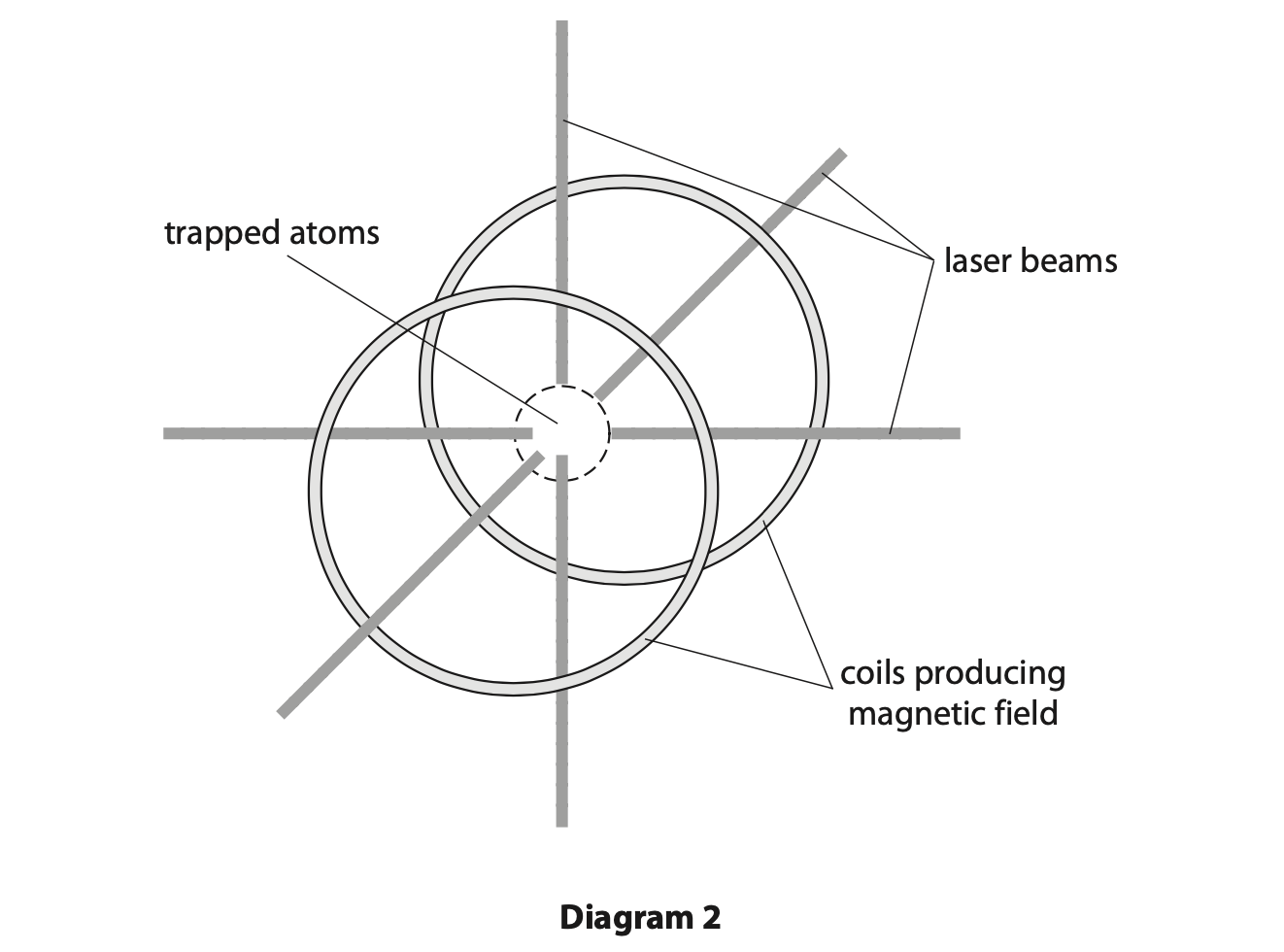
Each trapped atom has a mass of 5.0 × 10–27 kg and a mean speed of 73m/s.
Calculate the temperature of the trapped atoms.
[mean kinetic energy of an atom = 2.1 × 10–23 × temperature in kelvin] (4)
temperature = …………………………………………………….. K
(Total for Question 11 = 9 marks)
January 2023 Paper 1PR Q8
8 A student wants to determine the density of air using an irregularly-shaped balloon made of metal foil.
The balloon has a label stating that the volume of the balloon when full is 490 cm3.
This is part of the student’s method.
Step 1 measure the mass of the empty balloon
Step 2 fill the balloon with air
Step 3 measure the mass of the full balloon
Step 4 subtract the mass of the empty balloon from the mass of the full balloon.
(a) (i) Name the equipment the student could use to measure the mass of the balloon. (1)
(ii) Suggest how the student could improve the reliability of their data. (1)
(b) The table shows the student’s results.
| Mass of empty balloon in g | 15.00 |
| Mass of balloon when full of air in g | 15.61 |
| Volume of air in cm3 | 490 |
Calculate the density of air to 2 significant figures.
Give the unit. (4)
density = …………………………………………………….. unit ……………………………………………………..
(c) Describe how the volume of the balloon full of air could be measured using a large beaker and some water.
You may use a diagram to help your answer. (3)
(Total for Question 8 = 9 marks)
January 2023 Paper 2P Q2
2 The photograph shows an ice cube placed on a metal tile. The solid ice cube melts to become liquid water.
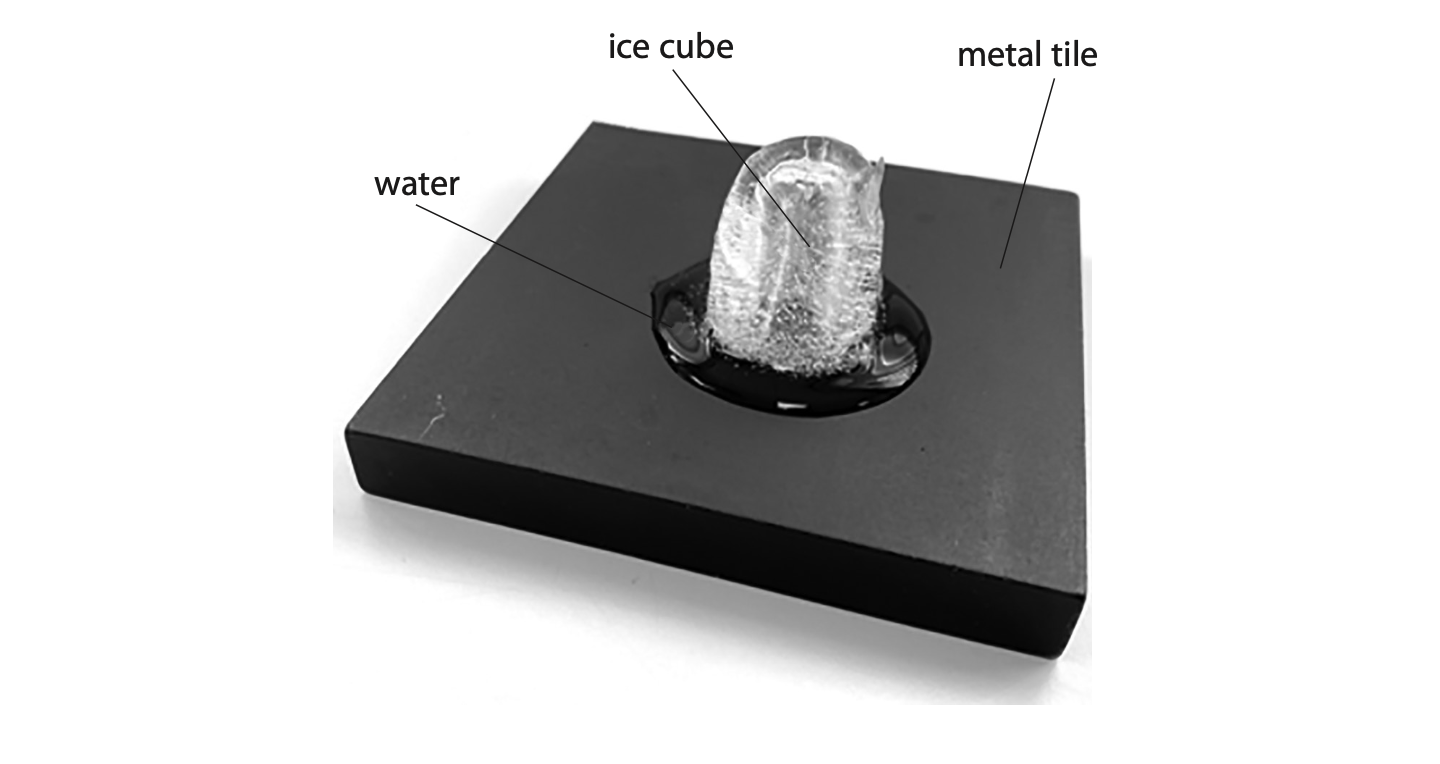
(a) Compare the arrangement of particles in a solid with the arrangement of particles in a liquid.
You may draw a diagram to help your answer. (3)
(b) Describe the difference in the movement of particles in a solid compared with the movement of particles in a liquid. (2)
(c) After the ice cube has melted, the liquid water increases in temperature. The water has a mass of 16 g and a specific heat capacity of 4200 J/kg °C.
Calculate the energy transferred to the liquid water as it increases in temperature from 3°C to 21°C. (3)
energy transferred = …………………………………………………….. J
(Total for Question 2 = 8 marks)
January 2023 Paper 2PR Q6
6. This question is about the use of water in central heating systems.
(a) A student does an investigation to find the specific heat capacity of water. This is the list of equipment they use.
- heater with a power output of 50 W
- power supply
- beaker
- water
- thermometer
- stopwatch
- connecting leads
- balance
Describe an investigation the student could use to find the specific heat capacity of water.
You may draw a diagram to help your answer. (5)
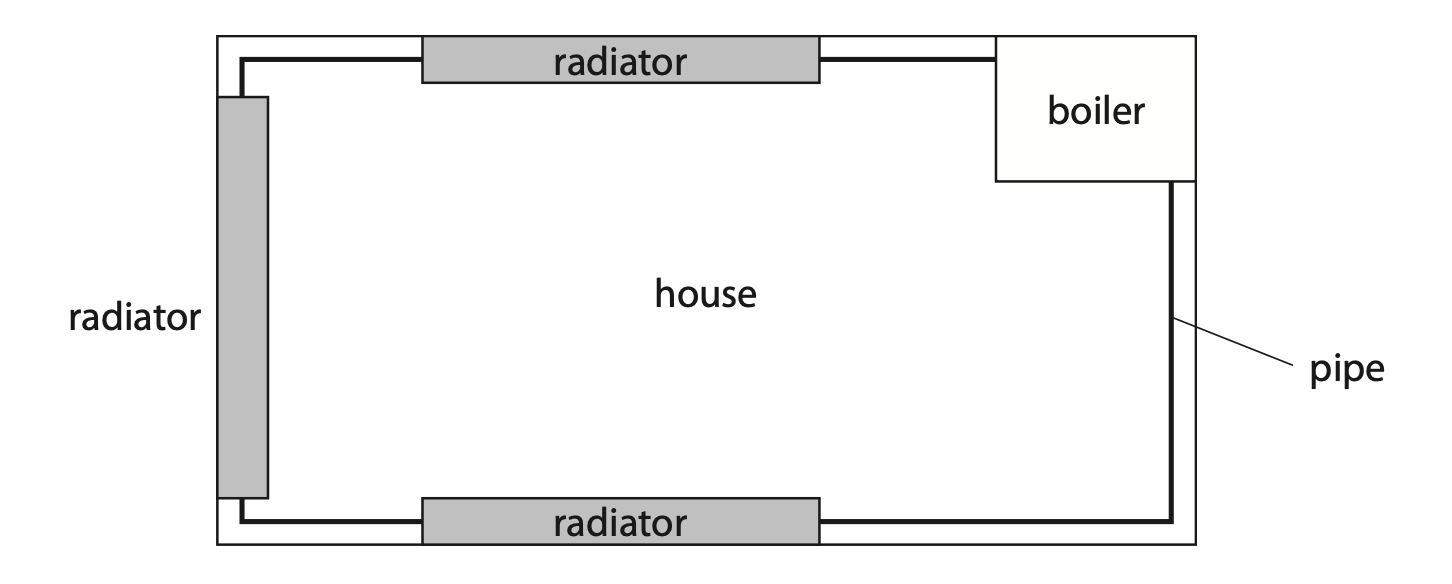
(b) The diagram shows a simplified central heating system viewed from above.
Pipes transport hot water around a house to radiators and back to the boiler.
The boiler heats water from 16 °C to 65 °C.
(i) Calculate the energy transferred from the boiler to 75 kg of water to raise the temperature of the water from 16 °C to 65 °C.
[for water, specific heat capacity = 4200 J/kg °C] (3)
energy = …………………………………………………….. J
(ii) The radiators transfer energy from the water to the air in the house.
The temperature of the water in the heating system decreases by 4 °C due to heat transferred to the air.
This causes the air in the house to increase in temperature by 15 °C.
The mass of air in the house and the mass of water in the heating system are approximately the same.
Explain why there is a larger temperature change in the air. (3)
(Total for Question 6 = 11 marks)
Jun 2023 Paper 1P Q9
9. A manometer is a device that can be used to measure the pressure difference between gas from a gas tap and the atmosphere.
When a gas tap is connected to the manometer, the liquid in the manometer moves due to the additional pressure of the gas.
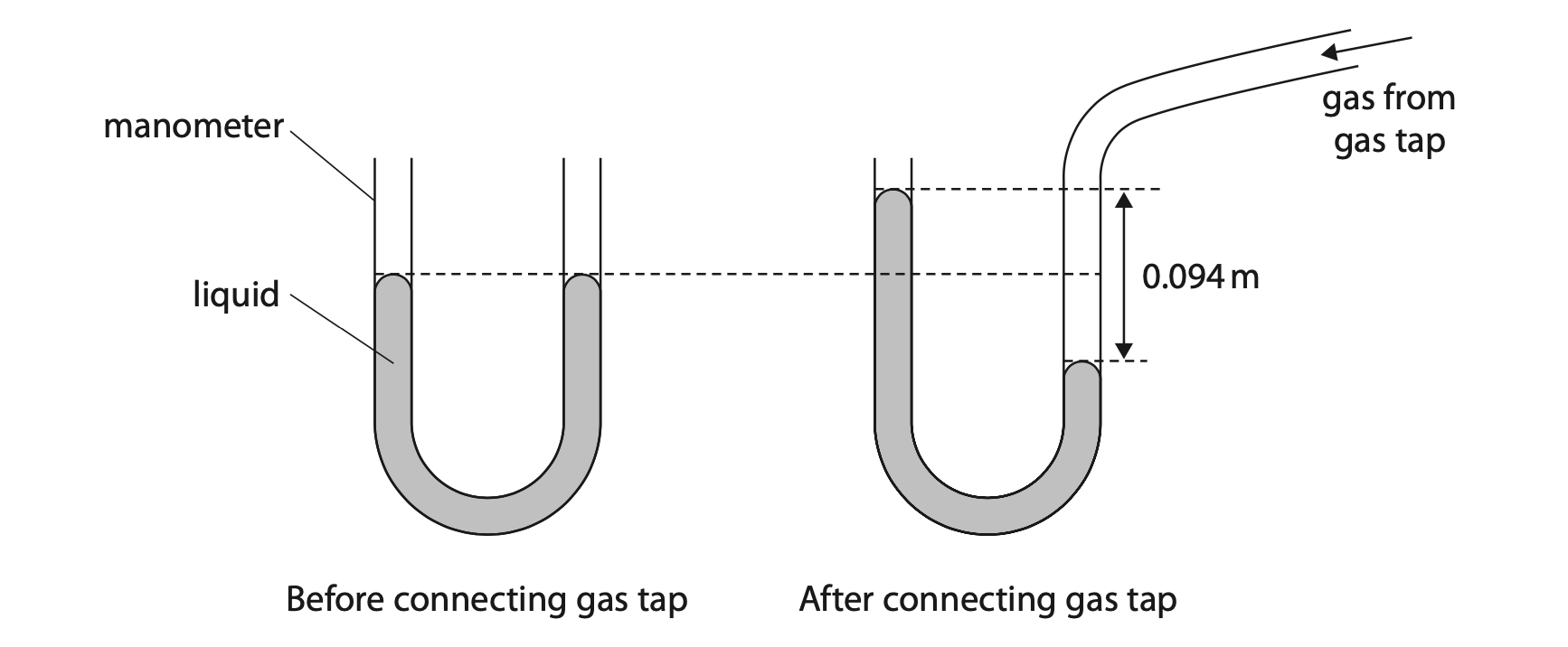
(a) The pressure difference is linked to the difference in height of the two surfaces of the liquid by the formula
pressure difference = density × g × height difference
The height difference between the two surfaces is 0.094 m.
Calculate the pressure difference between the gas from the gas tap and the atmosphere.
[for liquid, density = 14 000 kg / m3 ] (2)
pressure difference = …………………………………………………….. Pa
(b) The graph shows how the velocity of the surface of the liquid changes with time from when the gas tap is opened to when the water level stops moving.
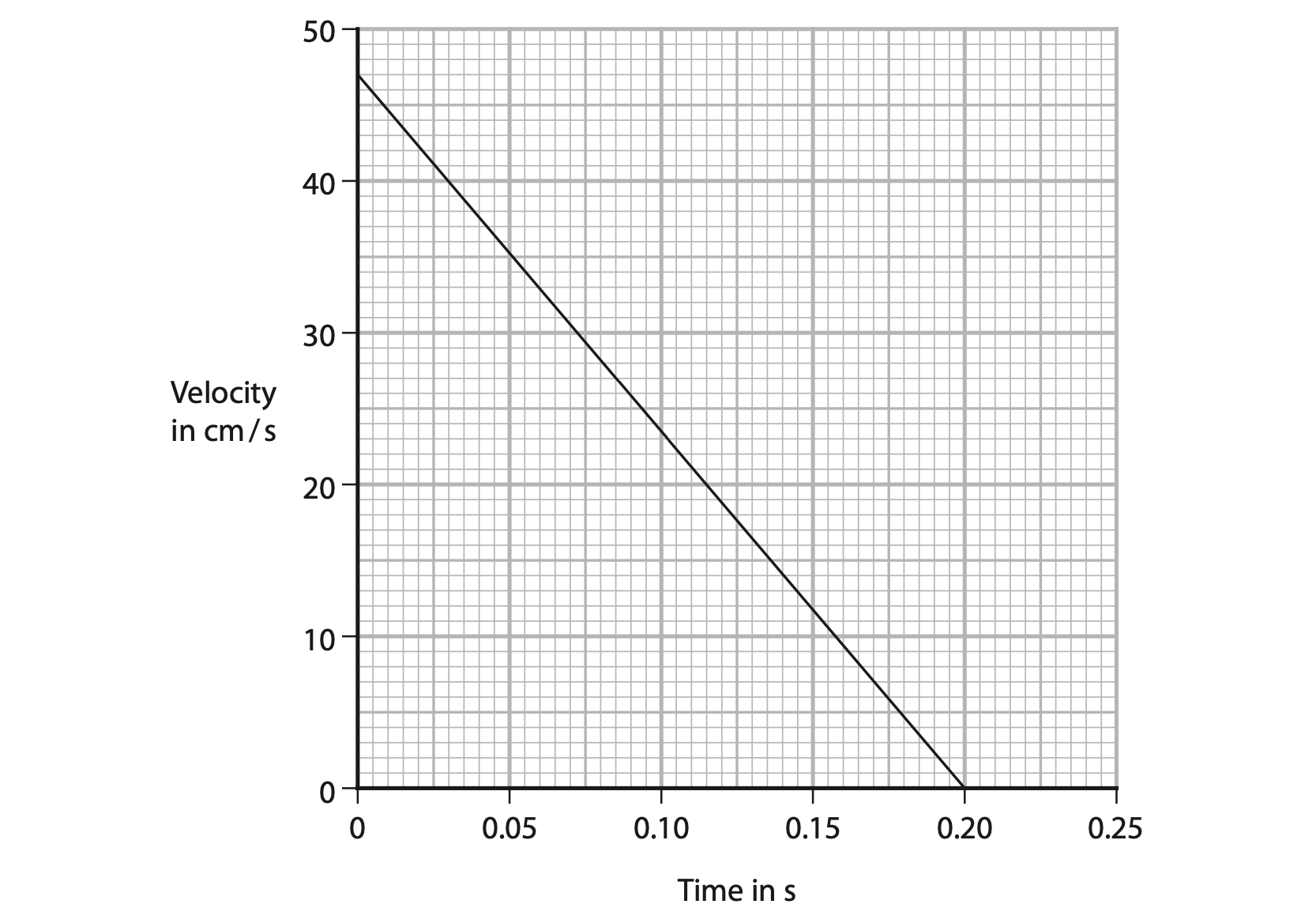
(i) Use the graph to show that the distance travelled by the surface of the liquid is 4.7 cm. (3)
(ii) Calculate the acceleration of the surface of the liquid. (3)
acceleration = …………………………………………………….. cm/s2
(c) Explain how the gas pressure changes if the temperature of the gas increases. You should use ideas about particles in your answer. (3)
(Total for Question 9 = 11 marks)
June 2023 Paper 2P Q2
2 A solid bar of chocolate is taken from a refrigerator.
(a) The temperature of the chocolate bar is 5°C
Describe the arrangement and motion of the particles inside the chocolate bar. (2)
(b) The chocolate is heated at a constant rate until the temperature reaches 45 °C.
The chocolate has a melting point of 32 °C and a boiling point of 55 °C.
(i) Describe the motion of the particles in the chocolate when the chocolate is at a temperature of 45 °C. (2)
(ii) Which of these is used to measure the temperature of the chocolate? (1)
A balance
B ruler
C stopwatch
D thermometer
(iii) Use the axes to sketch a graph of how the temperature of the chocolate changes with time when it is heated from 5 °C to 45 °C. (3)
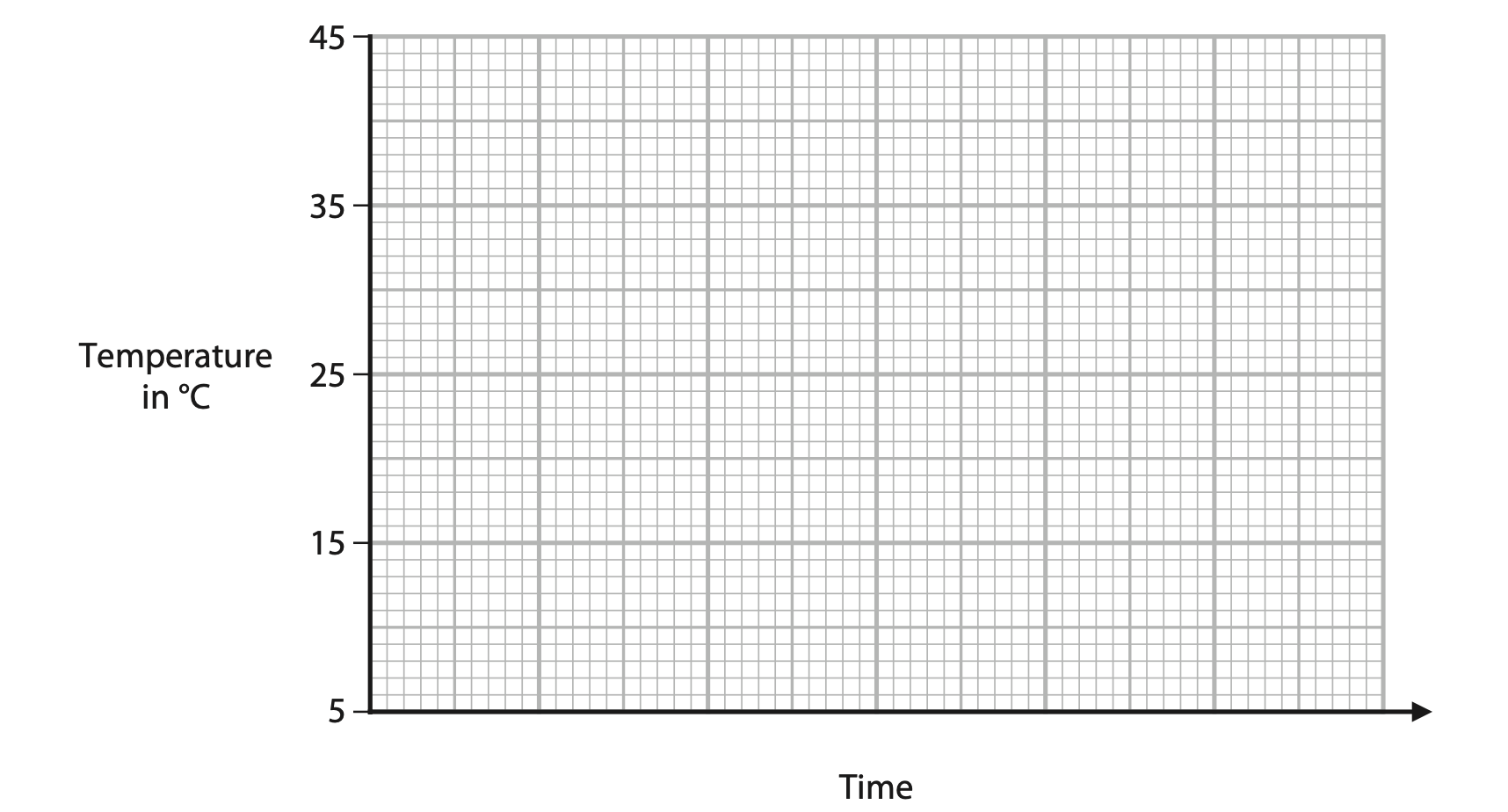
(Total for Question 2 = 8 marks)
Jun 2023 Paper 2P Q8
8 Concrete on top of buildings can be used to heat water.
The photograph shows a concrete and water heating system being built into the roof of a house.

(a) A scientist wants to determine the specific heat capacity of concrete.
The diagram shows some of the equipment they could use.
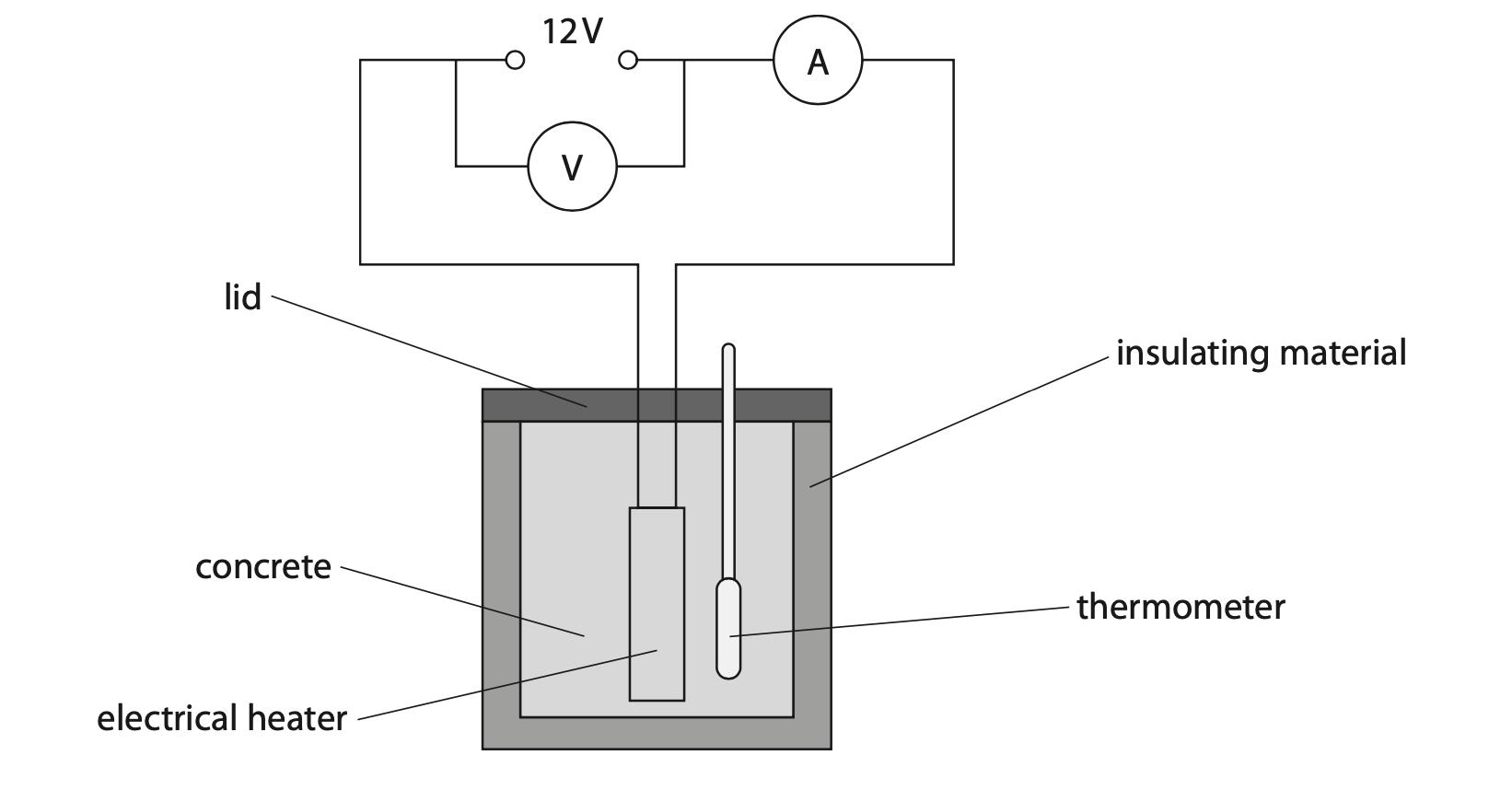
Describe a suitable method to find the specific heat capacity of concrete. (5)
(b) Explain the advantage of the concrete having a high specific heat capacity when it is used to heat water in the heating system. (2)
(Total for Question 8 = 7 marks)
June 2023 Paper 2PR Q5
5. This question is about specific heat capacity.
(a) State what is meant by the term specific heat capacity. (3)
(b) The diagram shows a sample of solid stearic acid being heated in a boiling tube using a water bath.
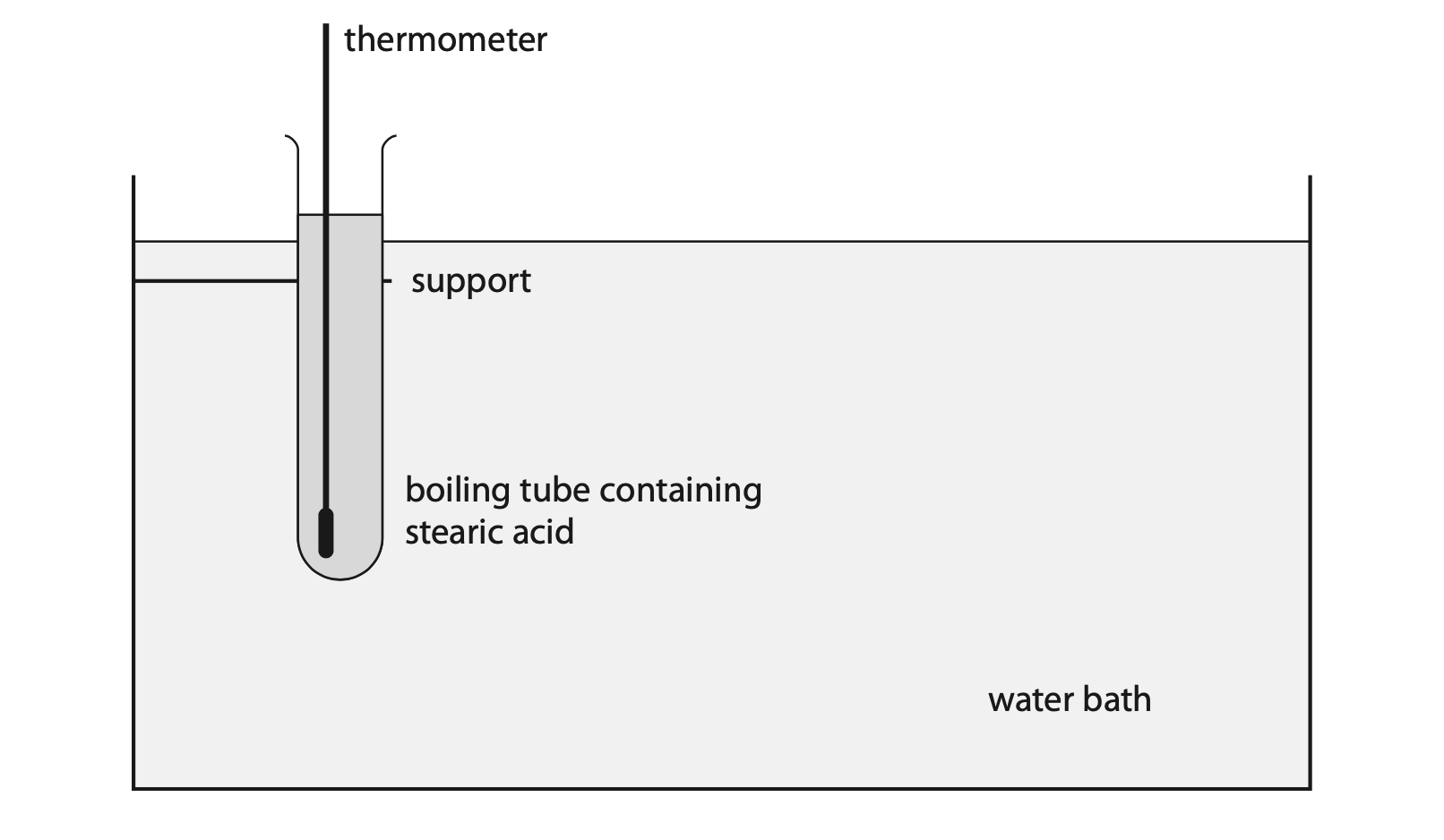
The mass of stearic acid in the boiling tube is 58 g.
When the boiling tube is placed in the water bath, the temperature of the stearic acid increases from 21 °C to 37 °C. The stearic acid does not melt.
As the temperature of the stearic acid increases, an additional 3500 J of energy needs to be transferred electrically to the water bath.
(i) Using this data, show that the specific heat capacity of the solid stearic acid is approximately 4 J / g °C. (3)
(ii) The true value for the specific heat capacity of solid stearic acid is 2.3 J / g °C.
Give a reason for the difference between the value in (i) and the true value. (1)
(Total for Question 5 = 7 marks)
November 2023 Paper 1P Q11
11 The diagram shows a simple barometer designed to measure atmospheric pressure.
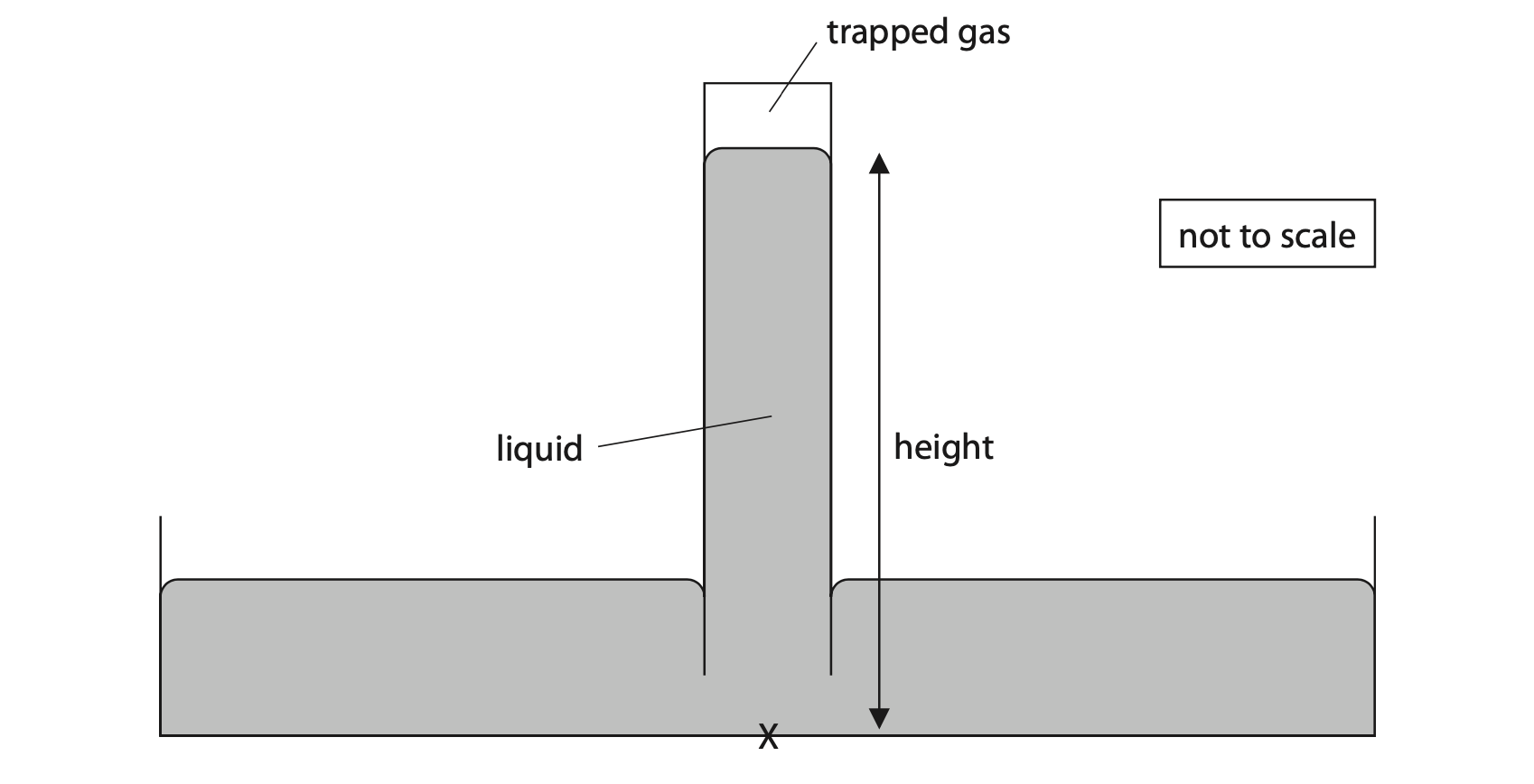
(a) (i) State the formula linking pressure difference, height, density and gravitational field strength, g. (1)
(ii) The total pressure at point X is 117 kPa.
The pressure of the trapped gas is 12 kPa.
Calculate the height of the liquid above point X. (4)
[density of liquid = 1.36 × 104 kg/m3]
height = …………………………………………………….. m
(b) The Sun shines onto the barometer, increasing the temperature of the trapped gas.
The volume of the trapped gas remains constant.
(i) Explain why the pressure of the trapped gas increases as its temperature increases. (3)
(ii) The temperature of the trapped gas increases from 23 °C to 38 °C.
The pressure of the trapped gas is 12 kPa when its temperature is 23 °C.
Calculate the pressure of the trapped gas when its temperature is 38 °C. (4)
pressure = …………………………………………………….. kPa
(Total for Question 11 = 12 marks)
Nov 23 Paper 1P Q5
5 A sample of steam (water in a gas state) is cooled using a very cold freezer.
(a) The steam is cooled from an initial temperature of 150 °C.
Calculate the initial temperature of the steam in kelvin (K). (1)
initial temperature = …………………………………………………….. K
(b) The temperature-time graph shows how the temperature of the steam changes during the cooling process.
The steam eventually becomes ice (water in a solid state).
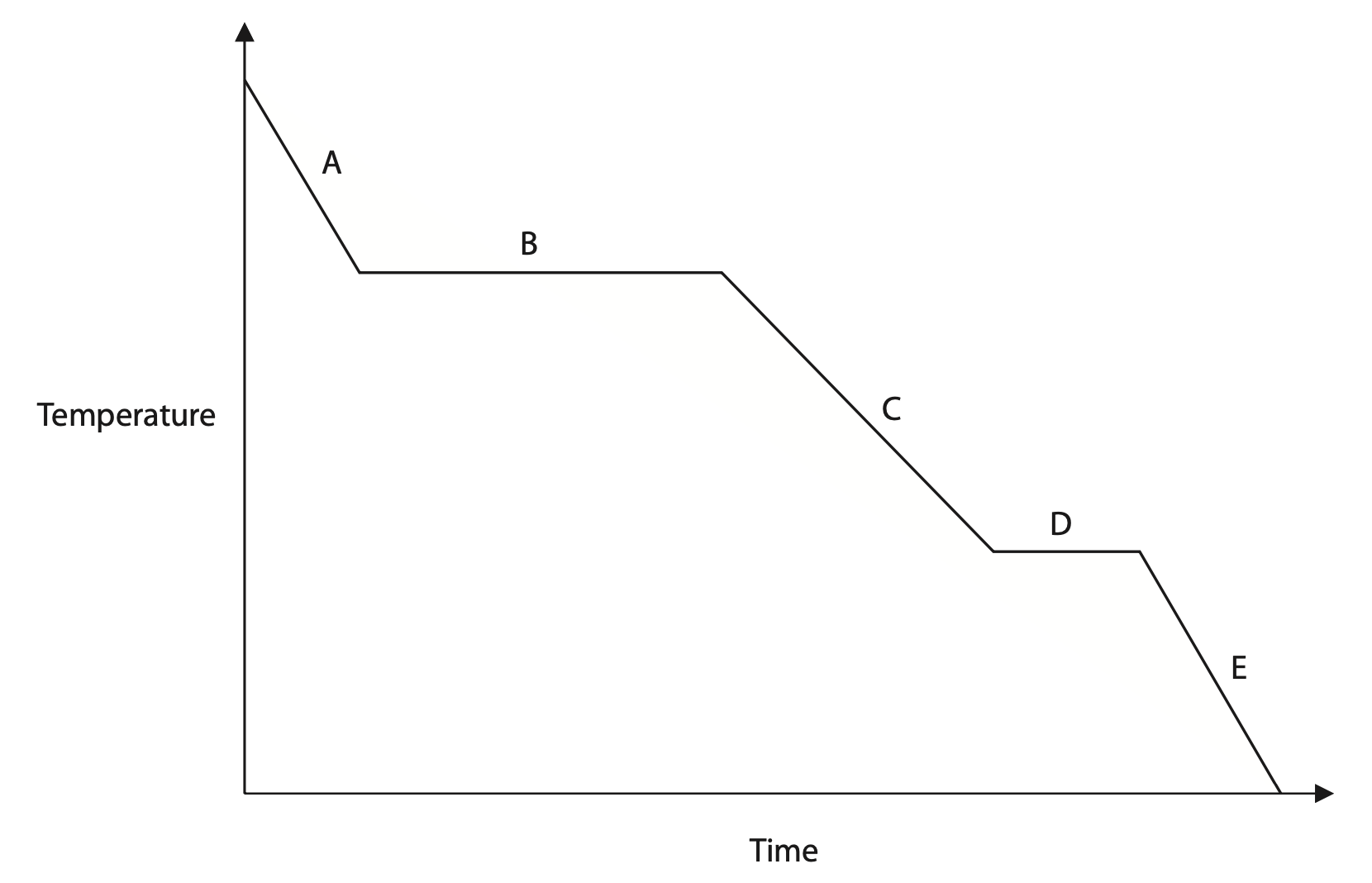
(i) The graph shows five stages, A, B, C, D and E, of the cooling process.
State which stages of the cooling process show a change of state. (1)
(ii) Describe the differences in the arrangement of particles when the sample is in a gas state (steam) and a solid state (ice).
You may draw a diagram to help your answer. (2)
(iii) Explain whether or not the energy in the kinetic store of the particles changes when the sample is changing state. (3)
(Total for Question 5 = 7 marks)









