IGCSE Physics Energy Resources and Energy Transfers Past Papers Exam Questions (Edexcel): 2019-20
We analysed the International GCSE past papers and grouped the questions by topic. Here, you will find questions relating to the topic – Energy resources and energy transfers. Use these to familiarise, practice and prepare for your IGCSE Physics examination.
You can find earlier papers below:
- IGCSE Physics Past Papers Exam Questions on Energy resources and energy transfers 2019-20.
- IGCSE Physics Past Papers Exam Questions on Energy resources and energy transfers 2021-22.
- IGCSE Physics Past Papers Exam Questions on Energy resources and energy transfers 2023-24.
What you need to know
Use the list below as a quick recap for what you need to know before attempting the past year exam questions under this topic. This is based on Edexcel International GCSE in Physics (4PH1) specification with first teaching Sept 2017 and first examination June 2019.
Paper 1 and 2: (4) Energy resources and energy transfers
Paper 1 covers all the topics except where it is marked “paper 2 only” while Paper 2 covers all topics.
A. Units
- kilogram (kg), joule (J), metre (m), metre/second (m/s), metre/second2 (m/s2), newton (N), second (s) and watt (W)
B. Energy transfers
- describe energy transfers from one energy store to another. Energy stores: chemical, kinetic, gravitational, elastic, thermal, magnetic, electrostatic, nuclear. Energy transfers: mechanical, electrical, heating, radiation (light and sound).
- principle of energy conservation.
- relationship between efficiency, useful energy output and total energy input. efficiency=useful energy transferred (output)/total energy supplied (input) ×100%
- describe a variety of everyday and scientific devices and situations for the transfer of the input energy including their representation by Sankey diagrams.
- describe how thermal energy transfer may take place by conduction, convection and radiation.
- explain the role of convection in everyday phenomena.
- explain how emission and absorption of radiation are related to surface and temperature.
- investigate thermal energy transfer by conduction, convection and radiation.
- explain ways of reducing unwanted energy transfer such as insulation
C: Work and power
- relationship between work done, force and distance moved in the direction of force. W=F×d
- work done is equal to energy transferred.
- use the definitions of work and power.
- the relationship for gravitational potential energy, mass, gravitational field strength and height. GPE = m × g × h
- the relationship for kinetic energy. kinetic energy = 1/2 × mass × speed2 (KE = 1/2 ×m×v2)
- how conversation of energy produces a link between gravitational potential energy, kinetic energy and work.
- describe power as rate of transfer of energy or the rate of work
- relationship between power, work done (energy transferred) and time. P = W/t
(paper 2 only)
D. Energy resources and electricity generation
- describe the energy transfers involved in generating electricity (wind, water, geothermal, solar heating, solar cells, fossil fuels, nuclear)
- describe the advantages and disadvantages of large-scale electricity production from various renewable and non-renewable resources.
June 2019 Paper 1 Q5
5 This is about stretching a spring
(a) The graph shows how the extension of a spring varies when a force is applied to the spring.
The line on the graph shows that the spring has been extended past its elastic limit.
The line has a straight section and a curved section.
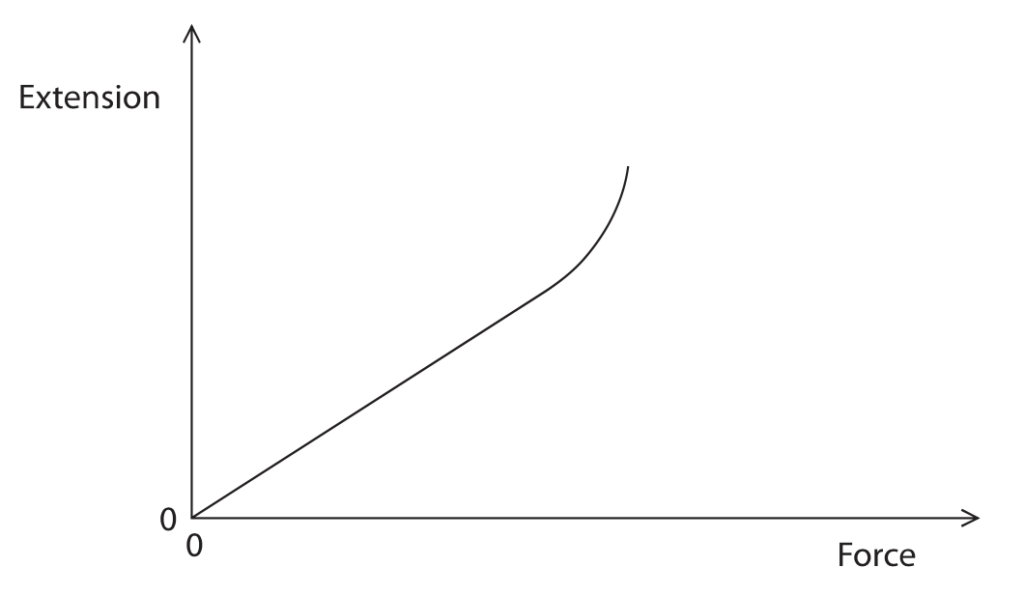
(i) Draw a cross on the line to show the elastic limit of the spring.(1)
(ii) Sketch another line to show how the extension will change when the force is decreased from its maximum value back to 0.(2)
(b) (i) State which energy store of the spring increases when it is stretched.
Assume the spring does not reach its elastic limit.(1)
(ii) How is this energy transferred to the spring?(1)
A electrically
B by heating
C mechanically
D by radiation
Total for Question 5 = 5 marks
June 2019 Paper 1 Q9
9 (a) The diagram shows a nuclear reactor.
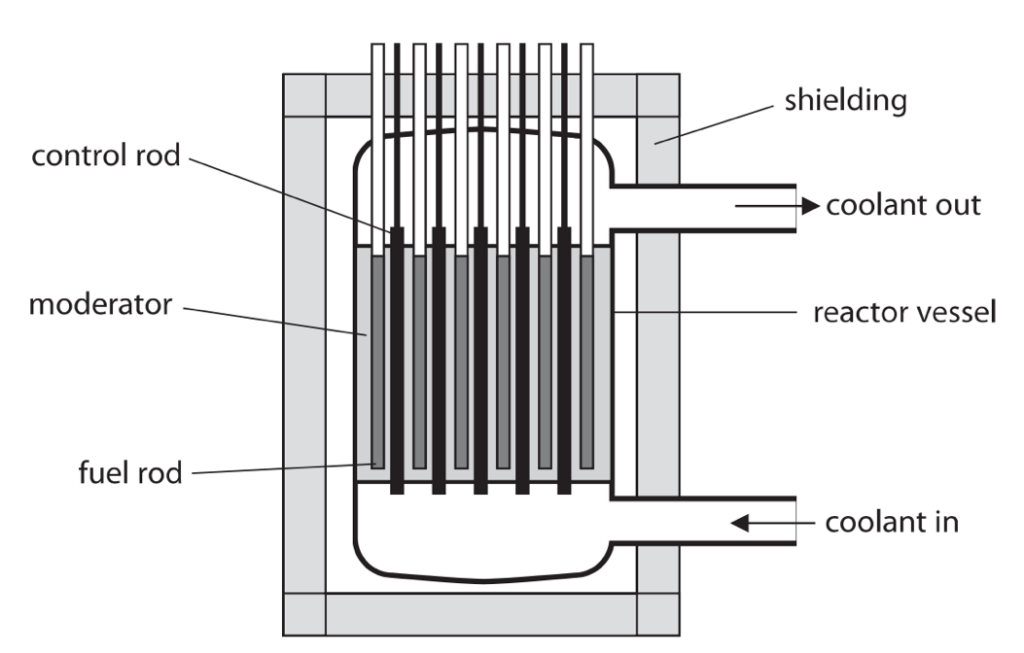
Each part of the nuclear reaction has a function and is made of a suitable material for its function.
Complete the table by giving the missing information. (5)
| Part | Function | Suitable material |
| control rod | boron | |
| moderator | graphite | |
| shielding | prevents irradiation of workers | |
| fuel rod |
(b) Heavy water is a compound of oxygen and an isotope of hydrogen called deuterium.
Deuterium is formed by the fusion of protons.
(i) State the meaning of the term isotope.(2)
(ii) Explain the difference between nuclear fission and nuclear fusion.(2)
(iii) State a location where nuclear fusion takes place.(1)
(iv) Explain why fusion cannot take place at low temperature or low pressure.(2)
Total for Question 9 = 12 marks
June 2019 Paper 1 Q12
12 (a) The diagram shows a ball of dough, of mass 580 g, held at a height of 92 cm above the floor.
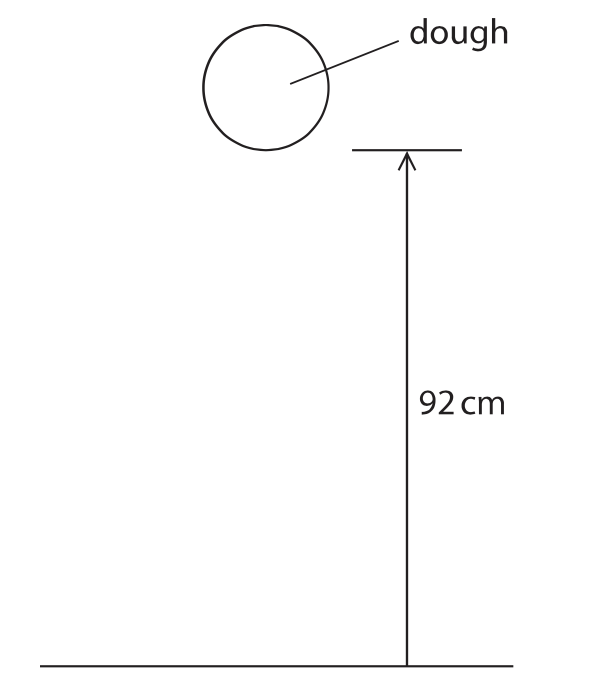
Calculate the increase in gravitational potential energy (GPE) stored in the ball of dough when it is above the floor.(3)
GPE = …………………………………….J
(b)The ball of dough hits the floor and does not rebound.
Describe the energy transfers taking place from when the dough is dropped to after it has hit the floor.(4)
You should refer to energy stores as well as transfers between energy stores at these stages.
- before the dough is dropped
- just before the dough hits the floor
- after the dough has hit the floor
Total for Question 12 = 7 marks
June 2019 Paper 1PR Q4
4 Photograph 1 shows an outdoor swimming pool.

(a) The water in the swimming pool is heated by the Sun during the day.
(i) State how energy is transferred from the Sun to the water. (1)
(ii) State what happens to the average speed of the water molecules as the water
is heated. (1)
(b) The water in the swimming pool cools down at night.
(i) Suggest why the water cools down at night. (1)
(ii) Photograph 2 shows the swimming pool with a plastic cover over the water.

Explain why the plastic cover reduces how much the water cools down at night. (4)
Total for Question 4 = 7 marks
June 2019 Paper 2 Q6
6 A dog sits on a water-filled bag to keep cool.

(a) The table shows some data about the dog and the water in the bag.
| Mass of water in kg | 8.7 |
| Power output of dog by heating in W | 75 |
| Specific heat capacity of water in J/kg °C | 4200 |
| Initial temperature of water in °C | 16 |
The dog sits on the bag for 22 minutes
(i) Calculate the energy transferred from the dog to the water by heating in 22 minutes.(3)
energy = …………………………………………………….. J
(ii) State an assumption you have made when calculating the energy transferred. (1)
(iii) Calculate the temperature of the water after 22 minutes. (4)
temperature = …………………………………………………….. °C
(b) Discuss why conduction is the main way that thermal energy is transferred from
the dog to the water. (3)
Total for Question 6 = 11 marks
June 2019 Paper 2PR Q1
1 (a) The diagram shows a fossil fuel power station.
Five stages of electricity generation and transmission are shown.
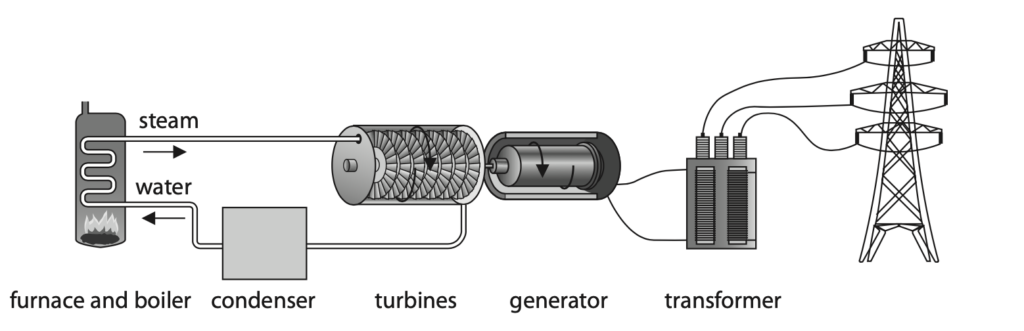
(i) The power station burns a fossil fuel in the furnace.
Give an example of a fossil fuel. (1)
(ii) Which stage of the power station transfers energy from a chemical store to a thermal store? (1)
A condenser
B furnace and boiler
C generator
D turbines
(iii) Which stage of the power station transfers energy electrically from a kinetic energy store? (1)
A condenser
B furnace and boiler
C generator
D turbines
(b) Electricity can also be generated by solar farms.

Energy is transferred from the Sun to the solar farm by radiation.
The solar farm uses photovoltaic cells to transfer this energy electrically.
Discuss the advantages and disadvantages of generating electricity using solar farms. (4)
Total for Question 1 = 7 marks
June 2019 Paper 2PR Q8
8 The photograph shows a device that can be used to ignite small pieces of combustible material.
The device consists of a cylinder containing trapped air and a piston.
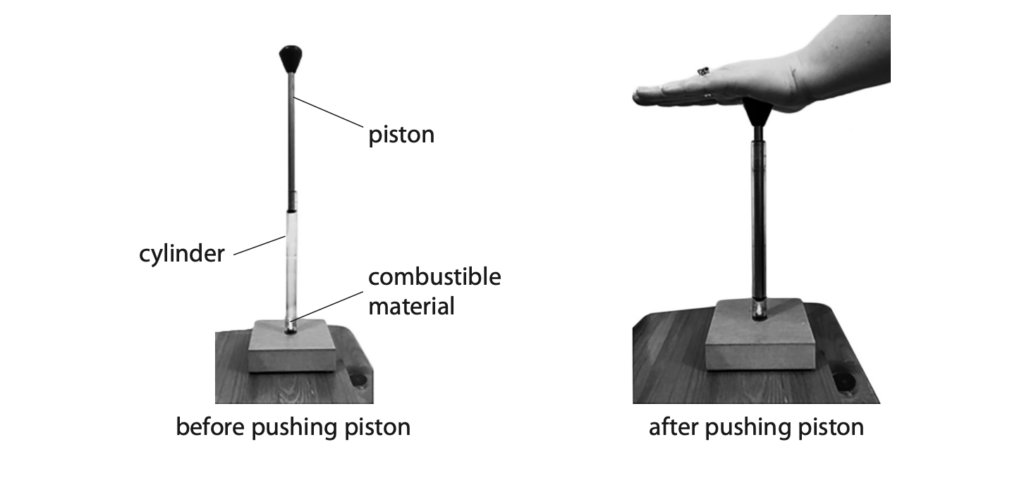
A small piece of combustible material is placed at the bottom of the cylinder.
When the piston is pushed quickly into the cylinder, the trapped air is compressed and heats up.
This increase in temperature is enough to ignite the combustible material.
(a) The piston is quickly pushed down a distance of 145 mm.
The average force exerted on the piston is 4.2N.
(i) State the formula linking work done, force and distance moved in the direction of the force. (1)
(ii) Calculate the work done on the trapped air when the piston is quickly pushed down. (2)
work done = ……………………………………….. J
(iii) State the maximum amount of energy that could be transferred to the trapped air. (1)
energy transferred = ……………………………………….. J
(iv) Using ideas about molecules, explain why the trapped air heats up. (2)
(b) Suggest why the trapped air does not reach a high enough temperature to ignite the combustible material if the piston is slowly pushed into the cylinder. (1)
Total for Question 8 = 7 marks
January 2020 Paper 1 Q6
6 A teacher makes a hot drink.
He puts the drink in a cup designed to keep the drink hot.
The photograph and cross-section diagram both show the cup.
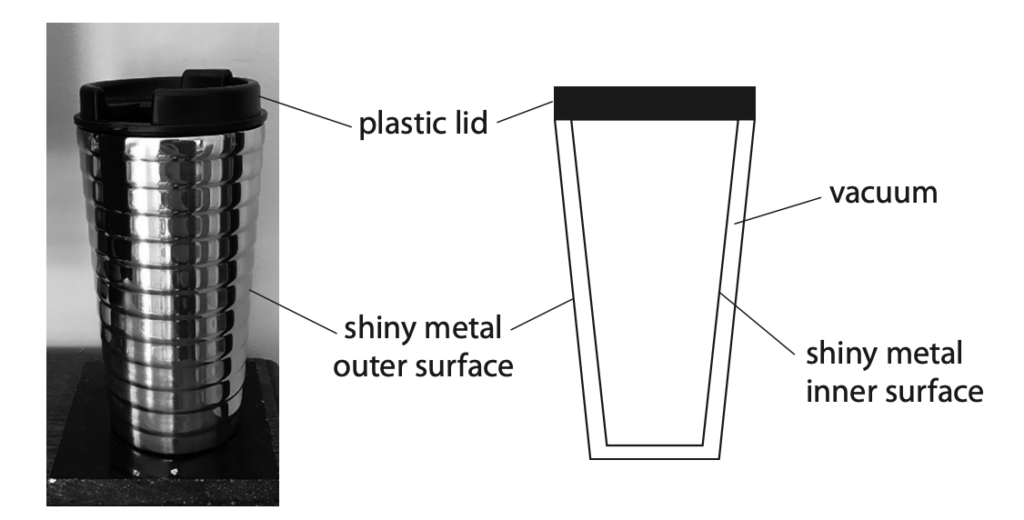
Explain how the design of the cup keeps the drink hot.
Refer to methods of energy transfer in your answer. (6)
Total for Question 6 = 6 marks
January 2020 Paper 2P Q1
1 This question is about energy resources.
(a) The table lists some methods of generating electricity using energy resources.
Place ticks (/) in the table to show if each method uses a renewable energy resource.
One has been done for you. (3)
| Method of generating electricity | Uses a renewable energy resource |
| coal power station | |
| diesel generator | |
| geothermal power station | |
| hydroelectric power station | |
| natural gas turbine | |
| nuclear power station | |
| solar cell | / |
| wind turbine |
(b) Solar cells can be used to generate electricity.
(i) How is energy transferred from the Sun to a solar cell? (1)
A by heating
B by radiation
C electrically
D mechanically
(ii) State one disadvantage of using solar cells to generate electricity. (1)
Total for Question 1 = 5 marks
January 2020 Paper 2PR Q4
4 A metalworker uses a hammer of mass 1.8 kg to deform a piece of metal on an anvil.
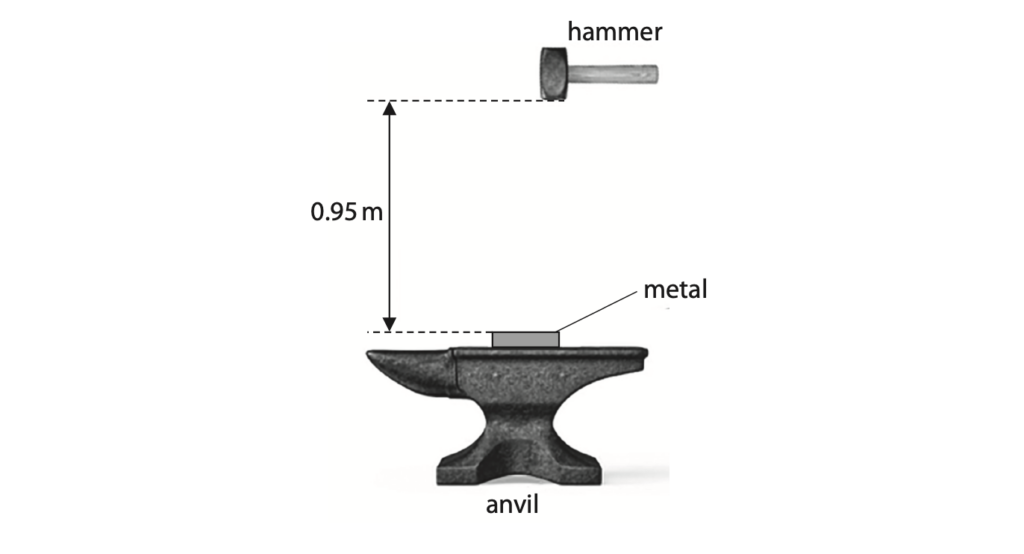
(a) (i) Calculate the increase in the hammer’s gravitational potential energy (GPE) store when the hammer is raised through a height of 0.95 m above the metal. (2)
[GPE gained = mass × gravitational field strength × height]
GPE increase = ………………………………………. J
(ii) The metalworker moves the hammer down to strike the metal.
The hammer must have at least 21 J of energy in its kinetic energy (KE) store as it hits the metal to deform it.
Explain why the metalworker must do at least 4 J of mechanical work on the hammer to deform the metal. (2)
(b) (i) Calculate the velocity of the hammer just before it strikes the metal. [KE of hammer = 21 J] (4)
velocity = ………………………………………. m/s
(ii) When the hammer hits the metal, the hammer comes to rest without rebounding.
Calculate the mean (average) force needed to bring the hammer to rest in a time of 0.12 s. (2)
mean force = ………………………………………. N
(Total for Question 4 = 10 marks)
June 2020 Paper 1PR Q5
5 A toaster is an electrical device used for toasting bread.

The toaster contains thin metal wires.
These wires get hot when there is a current in them.
The wires transfer energy to the bread by heating and by radiation.
(a) Give a reason why the wires in the toaster are connected in parallel. (1)
(b) (i) State the formula linking power, current and voltage. (1)
(ii) The power rating of the toaster is 2.8 kW.
Calculate the total current in the toaster. [mains voltage = 230V] (3)
current = ……………………………………………………………………….. A
(iii) The toaster contains 48 thin metal wires.
Calculate the current in each of the thin metal wires.
current = ……………………………………………………………………….. A
(Total for Question 5 = 6 marks)
June 2020 Paper 1PR Q7
7. The photographs show two different breeds of cat.

Cat X has no fur and light-coloured skin.
Cat Y has thick, black fur.
Both cats have the same body temperature and transfer energy to their surroundings when they are outside on a cold day.
Compare how cat X and cat Y transfer energy to their surroundings.
Refer to conduction, convection and radiation in your answer. (6)
(Total for Question 7 = 6 marks)
June 2020 Paper 2P Q4
4 The photograph shows a solar power farm.

(a) Discuss the advantages and disadvantages of using solar power rather than fossil fuels to generate electricity. (4)
(b) Solar panels produce direct current (d.c.).
The National Grid in many countries operates on alternating current (a.c.).
Describe the difference between direct current (d.c.) and alternating current (a.c.). (2)
(Total for Question 4 = 6 marks)
June 2020 Paper 2P Q6
6 The Parker Solar Probe is a spacecraft that was launched in 2018 on a mission to explore the Sun.
The spacecraft is partly covered in tiles.
These tiles are filled with a solid containing small pockets of trapped air.
The outer surface of the tiles is painted white.
These tiles protect the electric circuits in the spacecraft from extreme temperatures.
(a) The diagram shows one of these tiles in cross-section.
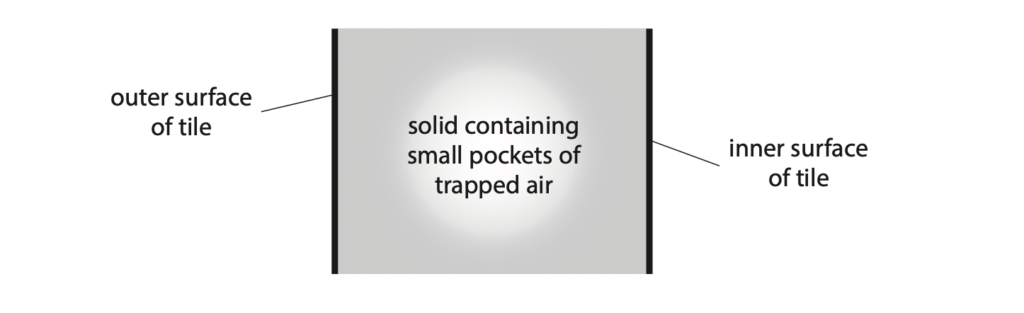
(i) Explain why the outer surface of the tile is painted white. (2)
(ii) Explain how the small pockets of trapped air help to reduce thermal energy
transfer from the outer surface to the inner surface of the tile. (2)
(b) The electric circuits inside the spacecraft are cooled by 4.5 kg of water.
The initial temperature of the water is 35 oC.
The thermal store of the water increases by 210 kJ.
Calculate the final temperature of the water. (4)
[specific heat capacity of water = 4200 J/kg oC]
final temperature = …………………………………………………….. oC
(Total for Question 6 = 8 marks)
June 2020 Paper 2PR Q5
5 Energy is transferred from the Sun to the Earth.
(a) Nuclear fusion happens in the Sun.
(i) Which energy store of the Sun decreases during nuclear fusion? (1)
A chemical
B kinetic
C nuclear
D thermal
(ii) How is energy transferred through space from the Sun to the Earth? (1)
A by heating
B by radiation
C electrically
D mechanically
(b) Solar panels use energy from the Sun to heat water.

(i) The total area of the solar panels is 15 m2.
Each 1.0 m2 of the solar panels receives 1000 J of energy per second from the Sun.
Show that the total energy transferred to the solar panels in 2 hours is about 100 MJ. (3)
(ii) A mass of 1100 kg of cold water is in the solar panels at an initial temperature of 20 °C.
Calculate the final temperature of this water after it has been heated by the Sun
for 2 hours. (4)
[specific heat capacity of water = 4200 J/kg °C]
final temperature = …………………………………………………. °C
(iii) Give a reason why the actual final temperature of the water will be lower than the calculated value. (1)
(Total for Question 5 = 10 marks)










