IGCSE Physics Past Years Exam Questions: Radioactivity and particles
We analysed the Pearson Edexcel International GCSE past papers and grouped the questions by topic. Here, you will find questions relating to the topic – Radioactivity and particles. Use these to familiarise, practice and prepare for your IGCSE Physics examination.
What you need to know
Use the list below as a quick recap for what you need to know before attempting the past year exam questions under this topic. This is based on Edexcel International GCSE in Physics (4PH1) specification with first teaching Sept 2017 and first examination June 2019.
Paper 1 and 2: (7) Radioactivity and particles
Paper 1 covers all the topics except where it is marked “Paper 2 only” while Paper 2 covers all topics.
A. Units
- becquerel (Bq), centimetre (cm), hour (h), minute (min) and second (s)
B. Radioactivity
- describe the structure of the atom in terms of protons, neutrons and electrons
- know the terms atomic(proton) number, mass (nucleon) number and isotope
- know that alpha (α) particles, beta (β–) particles and gamma (γ) rays are ionising radiations emitted from unstable nuclei in a random process
- describe the properties of α and ß particles and γ rays in terms of penetrating power and ability to ionise
- describe the effect on the atomic and mass number of nuclei caused by the emission of the four main types of radiation (alpha, beta, gamma and neutron radiation)
- understand how to balance a nuclear equation in terms of mass and charge
- know that ionising radiations may be detected by a Geiger-Müller tube or photographic film
- explain the sources of background (ionising) radiation from Earth and space
- know that the activity of a radioactive source decreases over time and is measured in becquerels (Bq)
- definition of the term half-life and use the concept to carry out calculations on activity, including graphical method
- uses of radioactivity in industry and medicine
- the difference between contamination and irradiation
- the dangers of ionising radiation: mutation in living organisms, damage cells and tissue, problems from the disposal of radioactive waste and how to reduce the risks.
C. Fission and fusion
- know that nuclear reactions, including fission, fusion and radioactive decay can be sources of energy
- that a nucleus of U-235 can be split by collision with a neutron (fission) and that the process releases kinetic energy
- that the fission of U-235 produces two radioactive daughter nuclei and a small number of neutrons
- describe how a chain reaction of fissions can be set up if the neutrons produced by one fission strike other U-235 nuclei
- describe the role played by control rods, moderator, and shielding in a nuclear reactor
- explain the difference between nuclear fission and nuclear fusion
- describe nuclear fusion as the creation of larger nuclei resulting in a loss of mass from smaller nuclei accompanied by a release of energy
- know that fusion is the energy source for stars
- explain why fusion does not happen at low temperatures and pressures, due to electrostatic repulsion of protons.
June 2019 Paper 1 Q3
3. This question is about food preservation.
(a) The diagram shows how gamma radiation is used to irradiate fruit stored in a wooden box.
The radiation kills bacteria on the fruit.
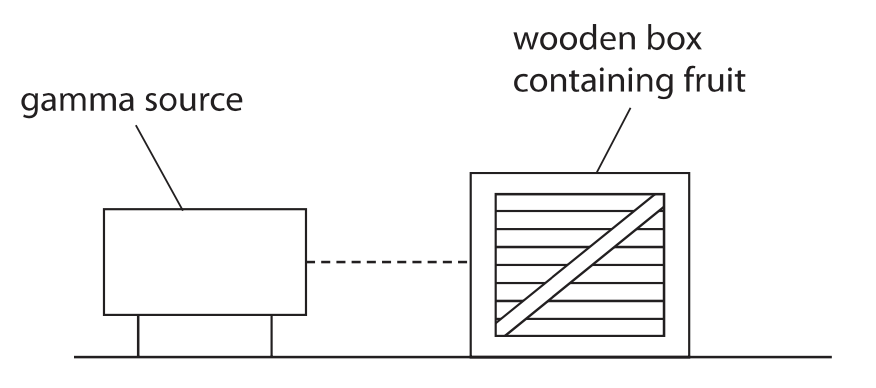
Explain why gamma radiation is used instead of alpha radiation to kill bacteria.(2)
(b) The Wooden box has this label

Explain why fruit irradiated with gamma radiation is safe to eat. (2)
(Total for Question 3 = 4 marks)
June 2019 Paper 1PR Q2
2. Some of the energy stored in the nuclei of atoms can be used to generate electricity.
(a) A nuclear fission power station generates electricity.
(i) State the role of the moderator in a nuclear fission power station (1)
(ii) Some of the daughter nuclei produced in nuclear fission are highly radioactive and emit high energy neutrons when they decay.
Explain which feature of a nuclear fission reactor reduces the risks from these high energy neutrons. (2)
(iii) The daughter nuclei can cause contamination and irradiation.
Describe the difference between contamination and irradiation. (2)
(b) Nuclear fusion is another process that could be used to generate electricity.
(i) Describe the process of nuclear fusion. (2)
(ii) State where nuclear fusion occurs naturally. (1)
(iii) Generating electricity from nuclear fusion is very difficult as the conditions needed are hard to achieve and maintain.
Explain the conditions required for nuclear fusion. (3)
(Total for Questions 2 = 11 marks)
June 2019 Paper 1PR Q3
3. Lead-210 is a radioactive isotope of lead and is represented using this symbol.
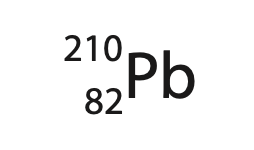
(a) State what is meant by the term isotope. (2)
(b) How many protons are in the nucleus of lead-210?
A 82
B 128
C 210
D 292
(c) (i) A sample of lead-210 has an initial activity of 240 Bq.
After 66 years, the activity of the sample is 30 Bq.
Calculate the half-life of lead-210. (2)
Half-life = …………………….years
(ii) Lead-210 decays into lead-206 through a number of stages.
This involves one alpha decay and a number of beta decays.
This incomplete equation summarises these stages.
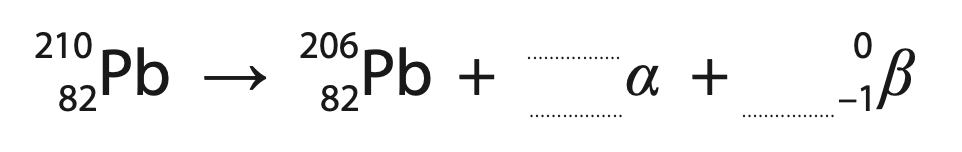
Complete the equation by giving the missing numbers.
Write your answers in the spaces provided. (2)
(Total for Question 3 = 7 marks)
June 2019 Paper 2 Q4
4. This is a question about alpha particles.
(a) Describe the nature of an alpha particle. (1)
(b) The diagram shows the path of an alpha particle as it passes close to a nucleus.
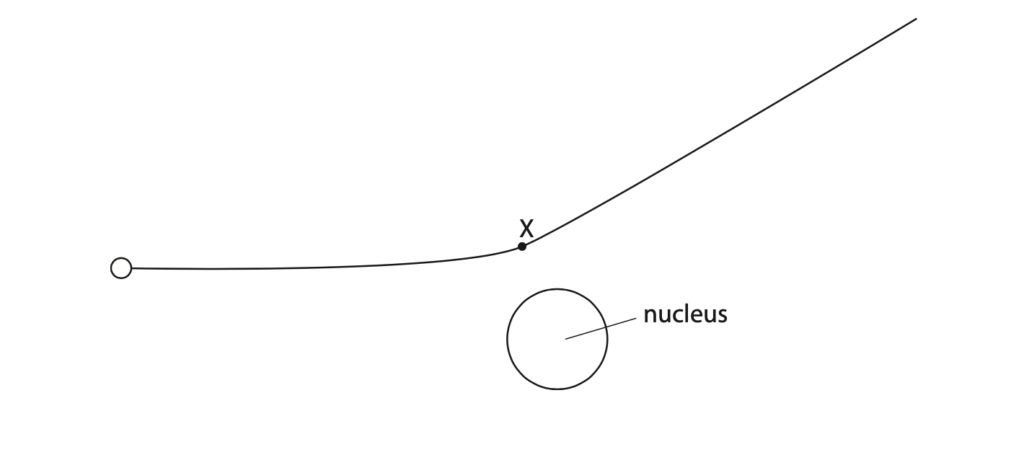
(i) Draw an arrow from point X to show the force on the alpha particle due to the nucleus.
Label this force Y. (2)
(ii) Draw an arrow to show the force on the nucleus due to the alpha particle.
Label this force Z. (2)
(iii) Explain how the path of the alpha particle shows whether the nucleus is positive, negative or neutral. (3)
(c) The alpha particle experiences a resultant force of 3.6N and has a mass of 6.6×10–27kg.
Calculate the acceleration of the alpha particle. (3)
Total for Question 4 = 11 marks
January 2020 Paper 1 Q11
11 Technetium-99m is an isotope of the element technetium.
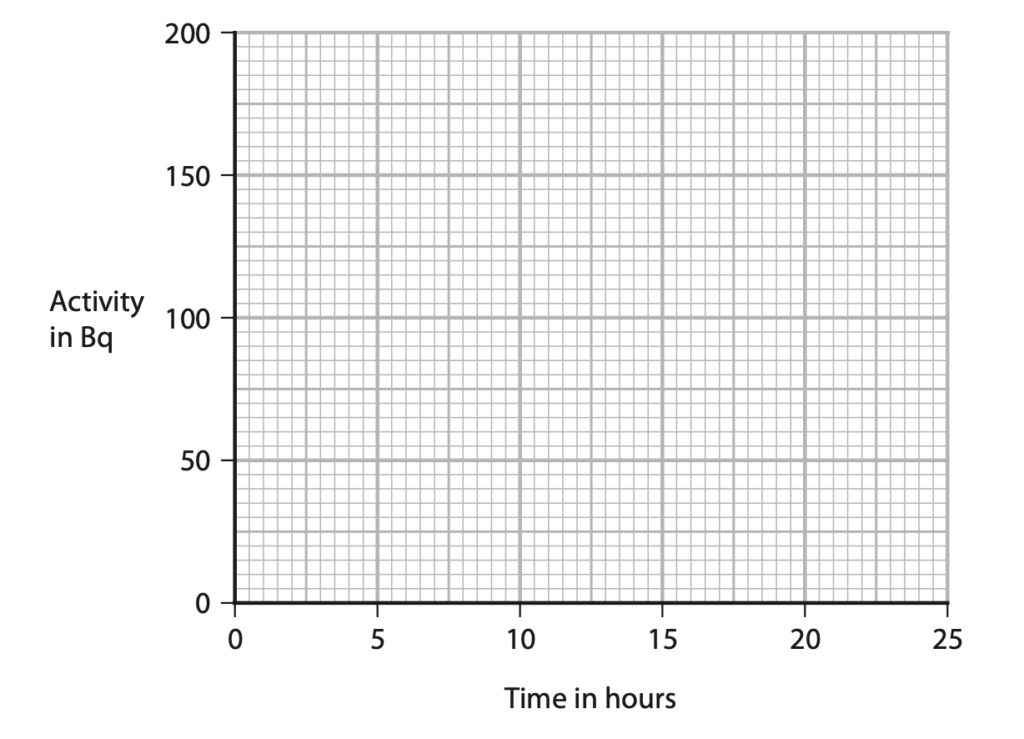
(a) Technetium-99m has a half-life of 6 hours.
A sample of technetium-99m has an initial activity of 160 Bq.
Complete the graph to show how the activity of this sample of technetium-99m changes over a period of 24 hours. (3)
(b) Technetium-99m has a half-life of 6 hours and can be used as a medical tracer.
It is injected into a patient’s blood and moves around the patient’s body.
Technetium-99m emits gamma radiation, which is used to locate the position of the tracer in the patient’s body.
(i) Technetium-99m does not exist naturally.
Suggest why technetium-99m is usually made at the hospital where it is used. (1)
(ii) Explain why technetium-99m is an effective isotope to use as a medical tracer. (2)
(c) The gamma radiation emitted by technetium-99m is potentially harmful to humans.
Discuss the risks of using technetium-99m to doctors and to patients. (3)
Total for Question 11 = 9 marks
January 2020 Paper 1PR Q9
9 A deioniser is a device used in rooms where workers build sensitive electronic circuits.
The deioniser contains a small block of polonium-210.
(a) (i) Complete the equation that shows the alpha decay of polonium-210 into lead-206.
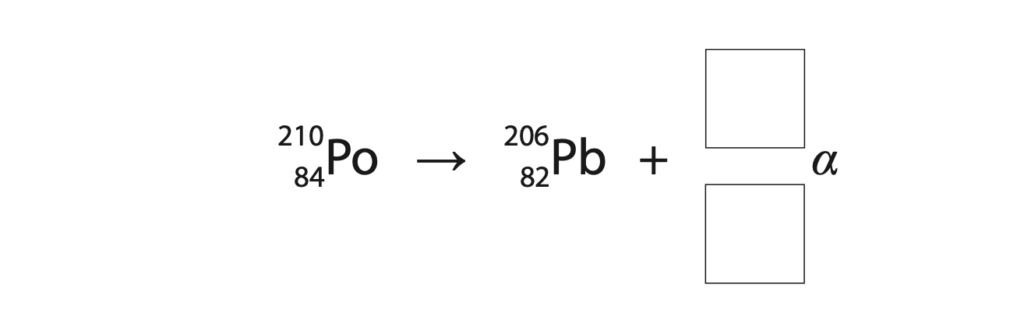
(ii) The alpha particle ionises air molecules in the room.
State what is meant by the term ionisation. (1)
(iii) Explain why workers in the same room as the deioniser are not at risk from the alpha radiation it emits. (2)
(b) The half-life of polonium-210 is 140 days.
(i) State what is meant by the term half-life. (2)
(ii) The initial activity of the polonium-210 source in the deioniser is 70 kBq.
Calculate the activity of the source after 420 days. (3)
activity = …………………………………………………….. kBq
(Total for Question 9 = 10 marks)
January 2020 Paper 2P Q7
7 The photograph shows a glass plate made from uranium glass.

Uranium oxide is used to give the glass a green colour.
(a) Uranium‐238 is the most common isotope of uranium and can be represented using this symbol.
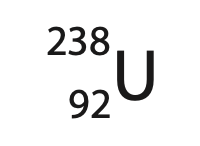
(i) State what information the numbers 92 and 238 give about the nucleus of this isotope of uranium. (2)
92
238
(ii) Uranium‐238 decays by alpha emission.
Describe how the nucleus of a uranium‐238 atom changes as a result of alpha emission. (2)
(b) The table gives some information about the uranium glass plate.
| mass of plate | 1.1 kg |
| percentage (%) of plate made of uranium‐238 (by mass) | 4.5% |
| mass of uranium‐238 atom | 4.0 × 10–27 kg |
(i) Calculate the number of uranium‐238 atoms in the plate. (2)
number of atoms = ……………………………………………………..
(ii) Uranium‐238 is an alpha emitter and has a half‐life of 4.5 billion years.
Explain why it is safe to eat food from the uranium glass plate. (3)
(Total for Question 7 = 9 marks)
June 2020 Paper 1P Q10
10 Technetium-98 and technetium-99 are isotopes of the element technetium.
(a) (i) Describe the difference between the nuclei of technetium-98 and technetium-99 (2)
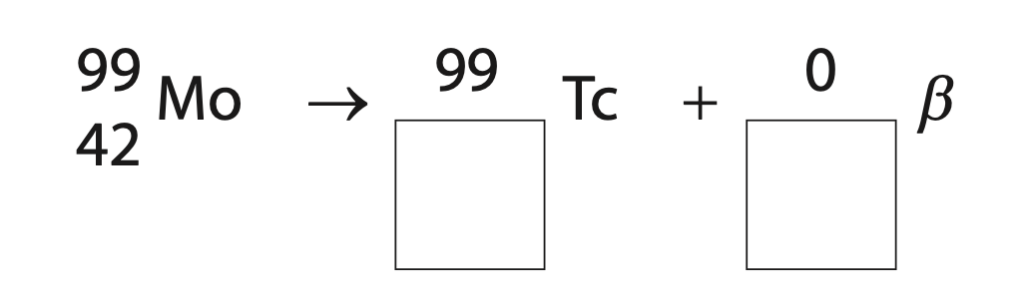
(ii) Technetium-99 is formed when the element molybdenum-99 decays.
Complete the nuclear equation for the decay of molybdenum into technetium-99 (2)
(b) Describe an experiment that a scientist could use to demonstrate that the emission from technetium-99 is gamma radiation.
Include details of a safety precaution in your answer. (5)
(c) A scientist measures the count rate at different distances from the technetium source.
The graph shows how the count rate changes with distance from the technetium source.
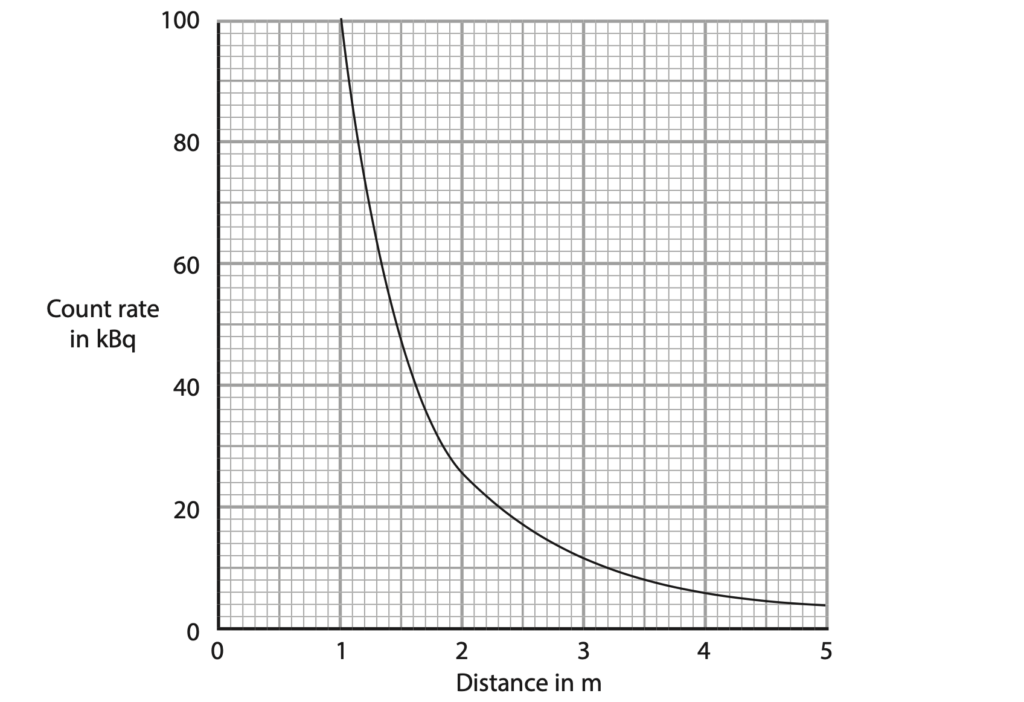
The scientist suggests that the relationship between the count rate and distance is
(distance)2 × count rate = constant
Use data from the graph to determine whether these results support this relationship. (4)
(Total for Question 10 = 13 marks)
June 2020 Paper 1PR Q2
2 This question is about a nuclear fission chain reaction.
(a) Uranium-235 is an isotope of uranium that can undergo nuclear fission.
The table gives some statements about this fission process.
Add ticks (✓) to the table to show which statements are correct. (3)
| Statement | Correct (✓) |
| uranium-235 loses a proton to become uranium-236 | |
| uranium-235 absorbs a neutron to become uranium-236 | |
| daughter cells are produced when uranium-236 splits | |
| the nuclear energy store of uranium-236 increases when it splits | |
| two or three neutrons are usually released when uranium-236 splits | |
| energy is transferred to the kinetic store of the fission products when uranium-236 splits |
(b) State which product of nuclear fission causes the next uranium-235 nucleus to split in the chain reaction. (1)
(c) Uranium-235 is radioactive and emits alpha radiation.
Which of these statements describes alpha radiation? (1)
A an electromagnetic wave
B a helium nucleus
C a high energy electron
D a high energy neutron
(d) Some of the products of nuclear fission are radioactive because their nuclei have too many neutrons.
These nuclei become more stable when a neutron changes into a proton.
State which type of radiation is emitted by radioactive nuclei with too many neutrons. (1)
(Total for Question 2 = 6 marks)
June 2020 Paper 2PR Q7
Magnetism and Electromagnetism and Radioactive
7 A machine called the synchrocyclotron (SC) was designed to cause protons to move in a circular path using strong magnetic fields.
(a) The SC used electromagnets to produce the strong magnetic fields.
Describe the construction of a simple electromagnet.
You may draw a diagram to support your answer. (3)
(b) The diagram shows a proton moving at velocity v in the magnetic field produced by the SC.

The direction of the magnetic field is into the page.
Using the left hand rule, determine the direction of the force acting on the proton due to the magnetic field. (1)
(c) The SC is surrounded by very thick concrete walls.
The radiation produced by the SC created atoms of radioactive barium-133 in the concrete walls.
Barium-133 has a half-life of 10.5 years.
(i) State what is meant by the term half-life. (2)
(ii) In 1990, the SC stopped being used at CERN, a research centre.
In 1990, the largest number of barium-133 atoms were found 40 cm deep in the concrete walls.
Since 2014, members of the public have been allowed to visit the SC at CERN.
Explain why the radioactive barium-133 in the concrete walls is not a risk to people visiting the SC. (2)
(Total for Question 7 = 8 marks)
January 2021 Paper 1P Q5
5 A teacher uses a radioactive source containing atoms of the isotope radium-226. (a) Give a safety precaution that would reduce the teacher’s exposure to radiation
when working with the radioactive source. (1)
(a) Radium-226 can be represented using the symbol
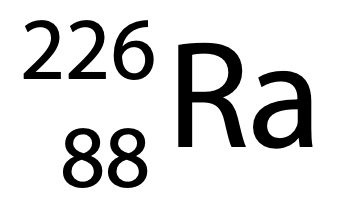
How many neutrons are in the nucleus of an atom of radium-226? (1)
A 88
B 138
C 226
D 314
(c) The teacher investigates the type of radiation emitted from the radioactive source.
(i) Give the name of a piece of apparatus that detects ionising radiation. (1)
(ii) The teacher finds that the radiation emitted from the radioactive source is not detected when the detector is more than 5 cm away from the source.
State the type of radiation emitted by the radioactive source. (1)
(d) The number of radium-226 atoms in the source decreases over time, with a half-life of 1600 years.
(i) State what is meant by the term half-life. (2)
(ii) The radioactive source contains 2.66 × 1021 atoms of radium-226.
Approximately how many atoms of radium-226 will remain in the source after 800 years? (1)
A 0.67 × 1021
B 1.33 × 1021
C 1.88 × 1021
D 2.66 × 1021
(Total for Question 5 = 7 marks)
January 2021 Paper 2P Q2
2 The nuclear equation shows how a nucleus of uranium-236 may undergo nuclear fission.
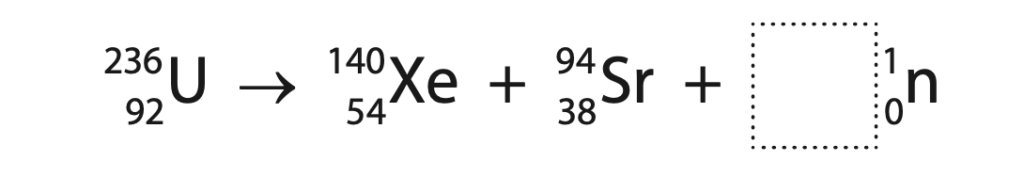
(a) Determine how many neutrons are released in this fission process. (1)
number of neutrons = ……………………………………………………..
(b) The table gives some of the constituents of this nuclear fission.
Complete the table by putting one tick (✓) in each row to show whether the constituent is a parent nucleus or a daughter nucleus. (2)
| Constituent | Parent nucleus | Daughter nucleus |
| strontium-94 | ||
| uranium-236 | ||
| xenon-140 |
(c) Describe how the neutrons released in this fission process can cause a chain reaction. (2)
(Total for Question 2 = 5 marks)
January 2021 Paper 1PR Q10
10 (a) Uranium-235 captures a neutron and undergoes nuclear fission in a chain reaction.
The equation shows a possible nuclear fission reaction.

Calculate x, the number of neutrons released by this fission reaction. (2)
x = ……………………………………………………..
(b) Describe what is meant by a chain reaction. (3)
(c) Iodine-129 is an isotope found in radioactive waste from nuclear power stations.
Iodine-129 has a half-life of approximately 15 million years.
A sample of iodine-129 has an activity of 72 kBq.
Show that the time required for the sample to have an activity less than 5 kBq is approximately 60 million years. (3)
(d) Some radioactive waste from nuclear power stations has a very long half-life. Discuss precautions that must be taken when disposing of this radioactive waste. (5)
(Total for Question 10 = 13 marks)
January 2022 Paper 1P Q2
2 A scientist wants to determine the half-life of a radioactive isotope.
The scientist measures the count rate from the radioactive isotope.
(a) State how the scientist should correct the count rate for background radiation. (1)
(b) The graph shows how the activity of the radioactive isotope varies with time.
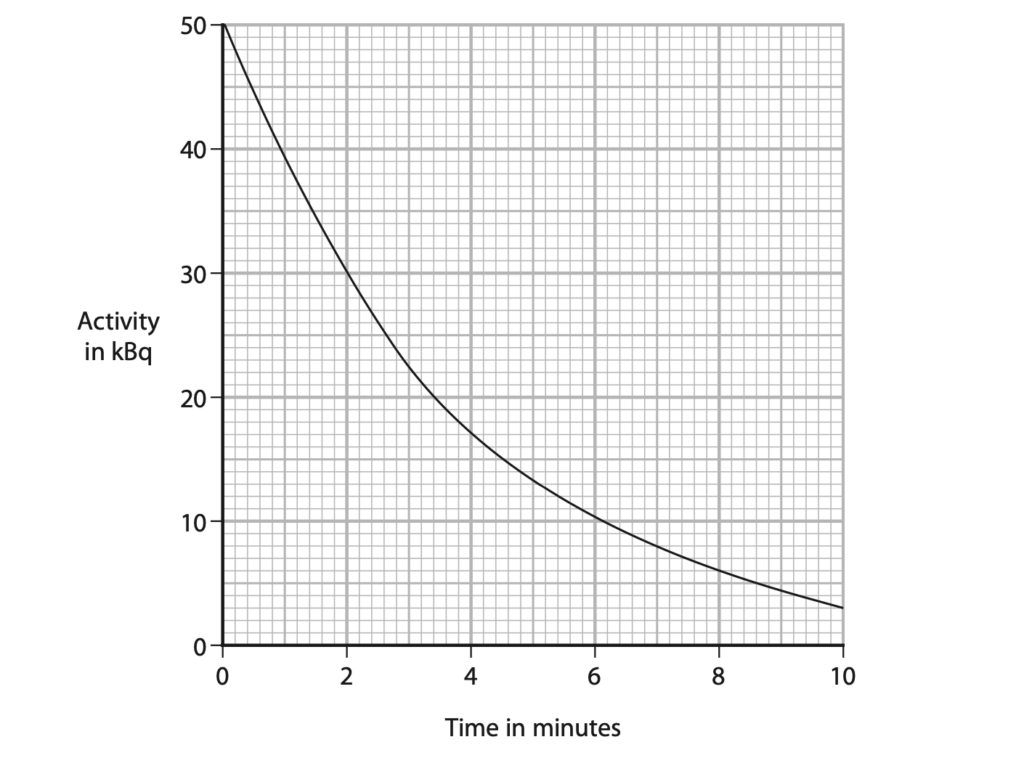
(i) Explain what is meant by the term half-life. (2)
(ii) Use the graph to determine the half-life of this isotope. (2)
half-life = …………………………………………………….. minutes
(Total for Question 2 = 5 marks)
January 2022 Paper 1PR Q1
1 (a) Bismuth‐207 is a gamma emitter and is represented by the symbol
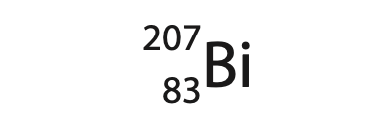
How many neutrons are in the nucleus of an atom of bismuth‐207? (1)
A 83
B 124
C 207
D 290
(b) Which of these gives the best description of gamma radiation? (1)
A a helium nucleus
B a high energy electron
C a high frequency electromagnetic wave
D a subatomic particle with mass, but with no charge
(c) A technician uses this method to investigate the penetration power of gamma radiation.
- place a gamma emitting source at a fixed distance from a radiation detector
- measure the count using the detector, for a period of one minute
- place a thin sheet of lead between the source and the detector
- measure the new count using the detector, for a period of one minute
- increase the number of lead sheets between the source and detector and repeat this process
The table gives some of the variables from the technician’s method.
Complete the table by placing a tick (✓) in each row to show whether each variable is an independent, a dependent or a control variable. (3)
| Variable | Independent variable | Dependent variable | Control variable |
| count measured using the detector | |||
| distance between source and detector | |||
| number of lead sheets | |||
| time period for measuring the count |
(Total for Question 1 = 5 marks)
January 2022 Paper 2PR Q5
5 This question is about nuclear fusion.
(a) The incomplete nuclear equation shows the fusion of two isotopes of hydrogen.
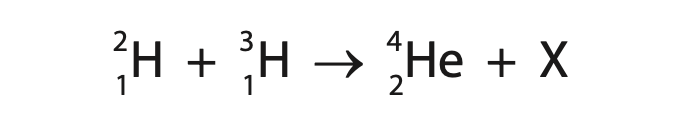
(i) Identify particle X in the nuclear equation. (1)
(ii) Each fusion reaction releases approximately 3 × 10–12 J of energy.
Estimate how many reactions are needed to produce 1 J of energy.
Give your answer to 1 significant figure. (2)
number of reactions = ………………………………………..
(iii) Explain why this fusion reaction will only happen at high temperature and high pressure. (3)
(b) A tokamak is an experimental nuclear fusion reactor.
Strong magnetic fields are used to contain the reactants inside the tokamak.
(i) The diagram shows a positively charged hydrogen nucleus in a uniform magnetic field directed out of the page.
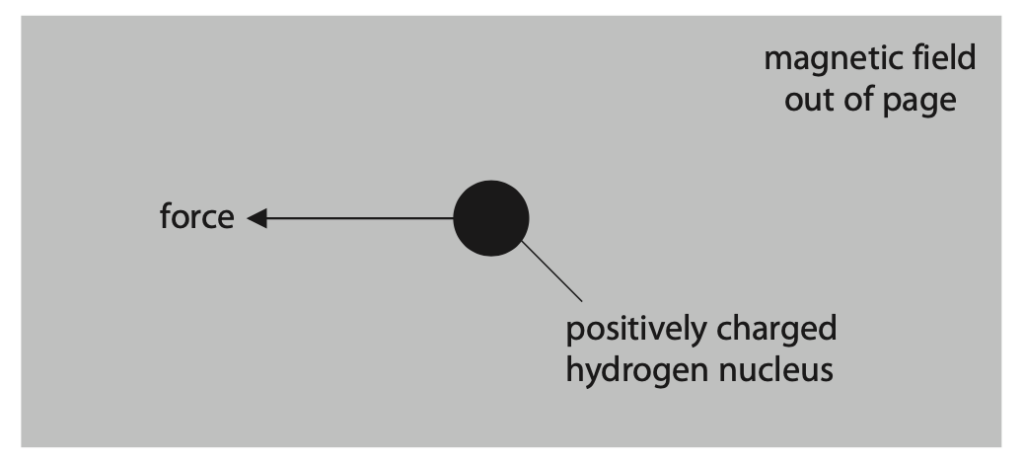
The nucleus experiences a force acting to the left because of the magnetic field.
Deduce how the nucleus must behave to experience this force. (2)
(ii) Suggest how engineers working on the tokamak could increase the force on the nucleus. (1)
(Total for Question 5 = 9 marks)








